Article Text
Abstract
Restenosis after percutaneous coronary interventions has been a major limitation of this otherwise very well-accepted method of coronary revascularisation. Coronary stents work by scaffolding the intimal flaps and preventing elastic recoil, which was a major problem after balloon angioplasty. The neointimal growth response to stenting contributes significantly to the restenotic process. Randomised studies comparing coronary artery bypass surgery with coronary stenting especially in multivessel disease clearly highlighted this problem. The problem has been greater in magnitude in special subgroups: diabetics, patients with small vessels (⩽2.5 mm in diameter), long segments of disease (⩾20 mm in length), etc. These limitations of Bare metal stents have been addressed by drug-eluting stents (DESs). Third-generation stents with bioabsorbable polymers like the Biolimus releasing Biomatrix stent have already become available in Europe and parts of Asia. A longer follow-up will prove their long-term safety vis-à-vis first-generation DES. The polymer-free stent with capability of using more than one drug, though very attractive, needs larger multicentric studies before gaining wider acceptance. The fully bioabsorbable stent is yet another promising concept. The feasibility has already been demonstrated, and finer refinements are under way. The future of newer DES thus is very promising, and most of the issues related to first-generation DES are at the threshold of being solved.
Statistics from Altmetric.com
Restenosis after percutaneous coronary interventions (PCI) has been a major limitation of this otherwise very well-accepted method of coronary revascularisation. Coronary stents work by scaffolding the intimal flaps and preventing elastic recoil, which was a major problem after balloon angioplasty. The neointimal growth response to stenting contributes significantly to the restenotic process. Randomised studies comparing coronary artery bypass surgery with coronary stenting especially in multivessel disease clearly highlighted this problem. The problem has been greater in magnitude in special subgroups: diabetics, patients with small vessels (⩽2.5 mm in diameter), long segments of disease (⩾20 mm in length), etc. These limitations of bare metal stents (BMS) have been addressed by drug-eluting stents (DESs).
These devices use antiproliferative drugs to limit exaggerated healing. DESs have been available since 2002 in Europe and Asia and from 2003 in the USA.
The first-generation DES in use are permanent polymer-based devices which release drugs to minimise neointimal hyperplasia in the segment of coronary artery subjected to PCI. The two drugs, which have been used widely, are: Sirolimus in Cypher (Cordis, Johnson & Johnson, Warren, New Jersey) and Paclitaxel in Taxus (Boston Scientific, Natick, Massachusetts). These drugs, used in very small doses, are released predictably by the polymer in the vessel wall within 2–4 weeks. Several randomised studies have proven the efficacy of both these stents up to a follow-up period of 4–5 years. The rates of repeat procedures for restenosis have declined to less than 10% from a figure of 25 to 40%. The property of drug induced delayed endothelialisation; acquired mal apposition and hypersensitivity reaction to the polymer in some autopsy specimens have highlighted the problem of late and very late stent thrombosis.1 These problems are somewhat unique to DES and not seen with BMS.
The magnitude of the problem of late and very late stent thrombosis has been calculated to be 0.2% per year in patients with on-label use and up to 0.6% per year for patients with off-label use (table 1) based upon the data from randomised studies and large registries.2 3 The new long-term follow-up data however have been more reassuring. The Settler network meta-analysis of 18 478 patients of all the randomised studies showed that all-cause death and cardiac death were no different when sirolimus eluting stent (SES), Paclitaxel eluting stent (PES) and BMS were followed up to 4 years.4 This, despite reports of higher late and very late stent thrombosis with first-generation DES, is explained by the fact that the restenotic events and increased need for repeat revascularisation rates in BMS offset the advantage of lower late thrombosis events.
A number of recent analyses in on-label and off-label indications for DES and propensity-based comparisons again confirmed the maintained superiority of DES over BMS without any increase in death and myocardial infarction (MI). The studies have been extended to indications like primary PCI in acute myocardial infarction (AMI). A number of studies5 6 including the most recent Horizons–AMI7 study have confirmed the safety and efficacy of DES for both Cypher and Taxus at least up to 1 year.
Despite the data allaying fears of higher myocardial infarction rates and death with the first-generation DES, there have been efforts to improve the safety and efficacy of the new-generation DES. The aim of this exercise is to improve the design in order to make newer DESs user-friendly and eliminate concerns because of polymer- and drug-related issues like late and very late thrombosis. The directions in which these issues are being addressed are listed in the box and are discussed below.
Safer drug–polymer combinations with thinner strut cobalt chromium platforms
New-generation stents made of chromium cobalt, with thinner struts, better deliverability and coated with newer and safer drugs and polymers have been developed. The animal data have shown a rapid and complete endothelialisation in these stents as compared with both Cypher and Taxus.
Endeavor (Medtronic, Galway, Ireland)
This is a phosphorylcholine-based cobalt alloy Driver coronary stent with phosphoryl choline as the polymer which elutes zotarolimus, a limus group of drug. In Endeavor I, a first-in-man (FIM) study, the 48-month Target Lesion Revascularisation (TLR) free survival was 96.9%, and the stent thrombosis rate was very low (1%). In the Endeavor II trial (Endeavor vs Driver), the major adverse coronary event (MACE) free survival rates at 3 years were 88.3% and 79.6% in the DES and BMS groups, respectively.8 The stent thrombosis rate was <1% at 3 years of follow-up.
The Endeavor III trial was a prospective randomised comparison of the Endeavor with the SES-Cypher. At 8 months, the Endeavor stent failed to meet its non-inferiority end point in terms of late lumen loss. The rates of death, MI and Target Vessel Revascularisation (TVR) were however similar in the two groups. The 2-year data of Endeavor III show a significant higher freedom from death and MI for Endeavor versus Cypher (97.8% vs 92.9%, p=0.015), indicating a superior safety profile for this DES. Endeavor IV, a pivotal US trial, showed that despite a higher late loss when compared with TAXUS, the target vessel failure rates were not different, making it non-inferior. The follow-up to 2 years is maintaining the safety end points. The stent is in use in Europe, Asia and also the USA. A prospective study comparing Endeavor vs Cypher (N=10 000) with stent thrombosis as the primary end point is in progress.
Box New-generation drug-eluting stents: directions of development
Safer drug–polymer combinations with new-generation thinner strut cobalt chromium platforms
Biodegradable polymers with special designs allowing multiple drug-delivery capabilities
Surface modification with drug delivery without use of polymer
Bioabsorbable stents
Despite the good safety record of the combined Endeavor data in terms of very low late thrombosis rates, there has been a concern of a late loss of more than 0.6 mm. A possible explanation for the lack of non-inferiority of the Endeavor is the rapid rate of drug elution in Endeavor. To obviate this limitation, a new formulation on the same carrier with the same drug, Zotarolimus, but with a new and different biostable Biolynx polymer has been made available. This combination slows down the drug release to 4–5 weeks. At angiographic follow-up, the late loss of this stent “Endeavor Resolute” is like other limus drugs in the range 0.2–0.25 mm. The initial clinical data of safety and efficacy are very promising, and the stent is already available for use in Asia and Europe. If the long-term results continue to be good and predictable, this stent is likely to replace the Endeavor.
Xience V/Promus (Abbot Vascular, Calif/Boston Scientific)
This is an Everolimus eluting cobalt chromium stent with a durable fluropolymer. The 14-day rabbit iliac artery model data showed 78% strut endothelialisation as compared with 38% with Endeavor, 20% with TAXUS and 7% with Cypher. This makes it a very promising stent from the safety point.
The SPIRIT I trial proved the efficacy of this stent over the new-generation BMS-Vision. The combined data of the SPIRIT II and III trials, which compared the Taxus and Xience V stents, showed that the in-stent late loss rates were significantly lower with Xience V (0.33 vs 0.14 mm, p=0.004). Likewise the 9-month ischaemic MACE was significantly (p=0.004) lower in the Xience V group (4.1%) than in the Taxus group (8.0%) indicating superiority in efficacy. The 2-year follow-up data maintain the initial results.
SPIRIT V, a large international registry of all-comers, is ongoing to assess the safety and efficacy in real-world cases. The 30-day MACE of 2.6% (Primary end point) are low and acceptable for this complex patient population. The long-term follow-up data of the 2663 recruited patients are awaited.
This stent has been in use all over Europe and Asia for more than 2 years now and has recently obtained US FDA approval also.
Biodegradable polymers with special designs
These new-generation stents have polymers that become degraded within a few months after deployment. The stent thus becomes a bare stent after 3–4 months, and polymer-related long-term problems are likely to be eliminated.
Conor MedStent and COSTAR stent (Conor MedSystems, Menlo Park, California)
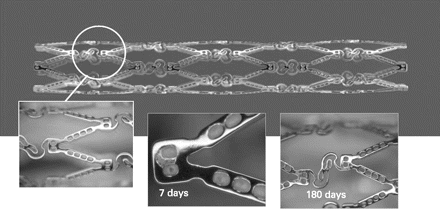
These are stainless steel and cobalt chromium stents respectively with reservoirs (wells) along the stent struts that are filled with a matrix of fully resorbable polymer (PLGA) and the drug Paclitaxel. The wells allow for potential unidirectional delivery of a drug (or drugs) to the luminal or mural sides of the vessel wall, as well as bidirectional delivery of the drug(s) to both sides simultaneously or different drugs to either side (fig 1).
COSTAR stent with hinge design and special wells to contain drug and bioabsorbable polymer. The polymer disappears from the wells in 180 days, as seen in porcine explants.
The major trials with this stent system PISCES (stainless steel) and COSTAR I9 (chromium cobalt) were dose-finding trials, while EuroSTAR and COSTAR II were two pivotal clinical trials. In the EuroSTAR trial, the 6- and 12-month death and MI rates were 1.4% and 2.1% each, TLR 1.7% and 2.9% and stent thrombosis 0.7% and 0%. The combined data of PISCES, COSTAR I, SCEPTER and EuroSTAR showed no case of late and very late stent thrombosis up to a period of 2 years' follow-up, highlighting the safety of this platform. The pivotal trial COSTAR II for US FDA approval however did not meet the non-inferiority criteria when compared with TAXUS. This difference was largely due to a significantly higher incidence of clinically driven TVR (8.1% vs 4.3%). The ambitious COSTAR programme was shelved following this study because of a lack of efficacy despite adequate safety.
Currently, the same platform is being utilised with sirolimus as the drug, and major trials are to be initiated by Cordis J & J.
Biolimus-A9 Eluting Stent (Biosensors International Group, Singapore)
Biolimus is a semisynthetic sirolimus analogue with 10-fold higher lipophilicity and the same potency as sirolimus. This drug in small doses, mixed with a polylactic acid (a biodegradable polymer), is coated only on the abluminal surface, minimising the dose of drug and the biodegradable polymer.
The Biomatrix stent was evaluated in a randomised non-inferiority study, where it has been compared with sirolimus eluting Cypher stent in all-comers.10 The study showed non-inferiority in the 9-month MACE (death, MI and clinically indicated TLR). The clinically indicated TLR was 4.4% for Biomatrix versus 5.5% for Cypher (p value for non-inferiority being 0.001).
This is the first study showing that a biodegradable polymer-based DES with Biolimus as the drug with unrestricted enrolment has a very acceptable and very low TLR, which is comparable with the existing first-generation stent. The long-term safety of this stent is expected to be good but needs confirmation at follow-up.
SUPRALIMUS (Sahajanand Medical Technologies, Surat, India)
Individual centre data are available, pertaining to this sirolimus-eluting indigenous stent which has a biodegradable polymer. SERIES I was an open label, non-randomised trial in which a total of 100 patients were recruited.11 The primary endpoints were MACE at 30 days and in-stent binary restenosis at 6 months, whereas the secondary objective was MACE at 9 months. Deaths, MI, TLR, stent thrombosis and MACE were 0.0% at 30 days. Death at 6 and 9 months was 2%. TLR at 6 and 9 months was 2 and 4%, respectively, bringing the MACE/restenosis rates to 45 and 6%, respectively, at the end of 6 and 9 months. The event-free survival at 9 months was 94%. Six-month QCA data revealed an in-stent late loss (mm), in-segment late loss (mm), restenosis (in-stent late loss, IS) (%), and restenosis (in-segment late loss, IL) (%) of 0.09 (SD 0.28), 0.02 (0.00), 0.0 and 1.7, respectively.
The company has an ambitious plan of having two more studies, SERIES II and SERIES III, which also include a head-to-head comparison with CYPHER.
Surface modification with drug delivery without polymer
Yukon (Translumina, Hechingen, Germany)
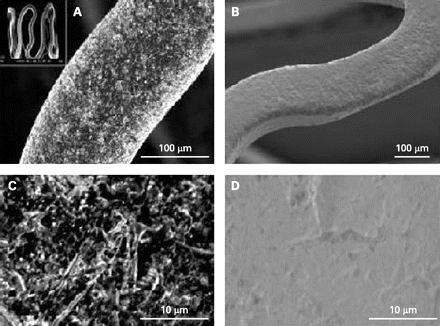
This is a polymer-free, microporus, Sirolimus-coated stent. The special stent surface known as Pearl has more than 1 million micropores per square centimetre of stent surface, which helps the drug to be adsorbed and spread uniformly on the stent surface, creating a smooth layer (fig 2). The drug is released predictably within 2–3 weeks, leaving a bare stent in place. The drug can be coated in the cardiac catheterisation laboratory using a specialised stent-coating machine. These stents can be used up to 4 months after coating as per the release patterns studied in vitro.
Scanning electron micrograph of the unique microporus Pearl surface Of the Yukon stent seen before and after coating with Sirolimus.
A two-centre randomised trial from Germany; ISAR test12 showed that the Yukon stent coated with 2% Sirolimus was not inferior to polymer-based TAXUS in the reduction of restenosis. The 2-year follow-up of this study shows no catch-up phenomenon in late loss for Yukon as compared with TAXUS.
The Indian TRANSIT13 registry with 1000 patients assessed the efficacy and safety of Yukon stent with polymer-free rapamycin coating in a real-world scenario. The primary end point of the study was the rate of target lesion reintervention for various patient cohorts at 1 year, while the secondary end points studied were the rate of thrombosis, AMI and cardiac death at 1 year. The stent thrombosis rates up to 2 years follow-up were <1% with an overall MACE rate of 4.6%.This registry confirmed the safety and efficacy of the non-polymer-based Sirolimus-coated Yukon stent on intermediate-term follow-up up to 2 years.
A recent randomised study14 using a combination of sirolimus and probucol coated stent without polymer when compared with fixed polymer DES, Cypher and Endeavor showed restenosis rates identical to Cypher and significantly lower than Endeavor (12%, 11% and 19% respectively (p=0.002 between Yukon, Cypher and Endeavor)). The late loss of Yukon and Cypher was similar, which was significantly lower than Endeavor. The polymer-less concept is very attractive, and the results need to be confirmed in larger multicentric studies. The stent is commercially available for use in Europe and several countries in Asia.
Bioabsorbable stents
The need for vessel scaffolding and drug delivery is temporary, and permanent scaffolding is superfluous after the vessel has completely healed. A bioabsorbable stent which would disappear completely over a period of time is a very attractive concept. The first stent to become available in this category was the Igaki–Tamai stent15 made of poly-l-lactic acid. The FIM study was carried out in a small cohort of 15 patients. The angiographic restenosis rate and TLR was 10.5%, which proved the stent's efficacy, safety and effectiveness of this stent. The concept theoretically should lead to lower late stent thrombosis, has a potential for reduced need for long-term dual anti-platelet therapy, facilitates treatment of in-stent restenosis and leaves the vessel without any foreign body in the long term.
Magnesium-based bioabsorbable stent
Magnesium, with its antithrombotic and antiproliferative properties, has been evaluated as the main component of a bioabsorbable stent. A recently reported FIM study using this stent in 63 patients across 10 centres reported no stent thrombosis, death or MI at the end of 1 year. The overall TLR was 47.5% at 1 year. Neointimal growth and negative remodelling were seen on IVUS. These results showed that magnesium-based bioabsorbable stents are safe, but the restenosis rates are unacceptably high, possibly because of an early dissolution in less than 4 months.16 Modifications of stent characteristics with prolonged degradation and drug elution are currently in development.
Everolimus eluting bioabsorbable stent
The stent has a bioabsorbable structure and polymer made of polylactic acid that controls the release of Everolimus. A recent FIM study in 30 patients with non-complex lesions “ABSORB Trial” using this stent revealed very promising results.17 At 30 days, the device and procedural success was very high: 93.5% and 100%, respectively. At 6 and 12 months, the MACE continued to be low at 3.3% with no cases of stent thrombosis. However, at 6 months, the angiographic late loss was 0.44 mm mainly due to a reduction in stent area as seen by intravascular ultrasound possibly due to recoil of the stent.
Changes in the stent design are under way to eliminate the problem of recoil. The new-generation stent will be under evaluation in due course. Bioabsorbable stents are at an early stage of development but hold considerable promise to overcome many of the limitations of the current generation of permanent metal implants.
Conclusion
The choice of DES with the availability of newer stents has allowed a wide range of choices. The first-generation DES: Cypher and Taxus have shown that restenosis rates are substantially reduced in several randomised studies and registries. The long-term data, however, brought out safety issues like late stent thrombosis. The second-generation stents, especially Xience V/PROMUS and Endeavor with safer polymer–drug combinations and more deliverable platforms, have started replacing the first-generation stents in the European, Asian and US markets. The safety and efficacy data up to 2 years appear very good. The third-generation stents with bioabsorbable polymers like the Biolimus- releasing Biomatrix stent have already become available in Europe and parts of Asia. A longer follow-up will prove their long-term safety vis-à-vis first-generation DES.
The polymer-free stent, with a capability of using more than one drug, though very attractive, needs larger multicentric studies before it can gain wider acceptance. The fully bioabsorbable stent is yet another promising concept. The feasibility has already been demonstrated, and finer refinements are under way.
The future of newer DESs thus is very promising, and most of the issues related to first-generation DESs are at a threshold of being solved.
References
Footnotes
Competing interests None.