Article Text
Abstract
Hypertrophic cardiomyopathy (HCM) is characterised by a thickened but non-dilated left ventricle in the absence of another cardiac or systemic condition capable of producing the magnitude of hypertrophy evident. It is the most common familial genetic disease of the heart (1/500 to 1/1000), as well as the most common cause of sudden cardiac death in young people and athletes. Survival rates of patients with HCM have improved from the 1960s onwards. Natural history in patients with HCM might vary from developing severe heart failure or atrial fibrillation, some die suddenly, often at a young age and in the absence of previous symptoms. Because of its heterogeneous clinical course and expression, HCM frequently presents uncertainty and represents a management dilemma to cardiovascular specialists and other practitioners.
Statistics from Altmetric.com
Hypertrophic cardiomyopathy (HCM) is characterised by a thickened but non-dilated left ventricle in the absence of another cardiac or systemic condition capable of producing the magnitude of hypertrophy evident (eg, aortic valve stenosis, systemic hypertension, and some expressions of athlete's heart)1 It is the most common familial genetic disease of the heart (1/500 to 1/1000),2 as well as the most common cause of sudden cardiac death in young people and athletes. Survival rates of patients with HCM have improved from the 1960s onwards, with earlier reports of 5–6% to less than 3%, in all-cause annual mortality, and a fall in annual sudden death mortality from 3% to 1%.3 Natural history in patients with HCM might vary from developing severe heart failure or atrial fibrillation; some die suddenly, often at a young age and in the absence of previous symptoms. On the other hand, it is also frequently compatible with normal longevity. Because of its heterogeneous clinical course and expression, HCM frequently presents uncertainty and represents a management dilemma to cardiovascular specialists and other practitioners, particularly those infrequently engaged in the evaluation of patients with this disease.4
Obstructive or non-obstructive cardiomyopathy
In the 1960s, Braunwald et al5 defined a specific disease process in which asymmetric septal hypertrophy, myofibril disarray and dynamic outflow tract obstruction were found. Thus, the early focus of hypertrophic cardiomyopathy was on this dynamic obstruction; the response of the obstruction to changes in preload, afterload, and contractility formed the basis for diagnosis. With the advent of echocardiography, which allowed direct visualisation of the hypertrophied myocardium, it became apparent that obstruction was not necessary for the diagnosis of hypertrophic cardiomyopathy, even though outflow tract obstruction was independently associated with an increased risk of both death (RR=1.6; p=0.02) and progression to NYHA class III or IV or death from heart failure or stroke (RR=2.7; p<0.001).6 HCM is now widely accepted as the preferred term, because it describes the overall disease spectrum without introducing misleading inferences that LV outflow tract obstruction is an invariable feature of the disease.
Based on ACC/ESC clinical consensus on hypertrophic cardiomyopathy, HCM was divided by its haemodynamic subgroups, based on the peaks gradient as assessed with continuous wave Doppler: (1) obstructive gradient under basal (resting) conditions ⩾30 mm Hg (2.7 m/s by Doppler), (2) latent (provocable obstructive), gradient <30 mm Hg under basal conditions and ⩾30 mm Hg with provocation and (3) non-obstructive, <30 mm Hg under both basal and provocable conditions.4
Despite its obvious impairment on diastolic function, the high contraction load imposed by the obstruction significantly worsens ventricular filling and relaxation. Other mechanisms by which obstruction produces symptoms are due to limitation of cardiac output, increased myocardial oxygen demand, and decreased coronary perfusion pressure. It is likely that greatly elevated left ventricular pressures created by obstruction lead to increased wall stress, myocardial ischaemia, and eventually, cell death and replacement scarring. This cellular remodelling, in turn, probably increases the likelihood that the left ventricle will become stiff and non-compliant, leading to diastolic dysfunction, and may also increase susceptibility to electrical instability and sudden death.6 7 In addition, obstruction is associated with distortion of the mitral valve apparatus, resulting in secondary mitral regurgitation, further elevating left atrial pressure, and contributing substantially to severe symptoms of dyspnoea.8 However, it seems that the likelihood of severe symptoms and death related to outflow tract obstruction did not increase as the gradient increased above the threshold of 30 mm Hg.6
Clinical course for HCM
For the majority of patients, the course is relatively benign, although disease-related complications can develop at any time and typically variable. A minority are at risk of serious complications including ventricular arrhythmia, sudden death, thromboembolism, congestive cardiac failure, heart block, and infective endocarditis. One study by Maron et al showed that 23% patients achieved normal life expectancy (>75 years old). Most patients (47%) experienced no or only mild limiting symptoms and lived virtually their entire lives with few HCM-related clinical consequences.9
In general, adverse clinical course proceeds along one or more of several of the following pathways, which ultimately dictate treatment strategies: (1) high risk for premature sudden and unexpected death; (2) progressive symptoms largely of exertional dyspnoea, chest pain (either typical of angina or atypical in nature), and impaired consciousness, including syncope, near-syncope or presyncope (ie, dizziness/lightheadedness), in the presence of preserved LV systolic function; (3) progression to advanced congestive heart failure; and (4) complications attributable to AF, including embolic stroke.1 10–12
Management
Because hypertrophic cardiomyopathy is a relatively rare condition, no proven therapy exists for HCM because no appropriate clinical trials have been performed. Selection of treatment relies principally on retrospective studies and clinical experience.4
A fundamental goal of treatment in HCM is the alleviation of symptoms related to heart failure.13 14 Since no data indicate that pharmacological therapy may change the course of the disease, treatment is generally not required in low-risk asymptomatic patients. Once the diagnosis is made, the patient's family history (data regarding the presence of hypertrophic cardiomyopathy or sudden death) should be carefully obtained. First-degree family members should undergo periodic screening with echocardiography every 5 years for this autosomal dominant disorder, and may not be appreciable until the sixth to seventh decade of life. Annual screening is recommended for adolescents 12–18 years of age. In the future, the diagnosis of hypertrophic cardiomyopathy may be based on the identification of mutations in the genes encoding the sarcomeric proteins, but this technique is not currently the standard of care. Patients should undergo an evaluation that includes 48 h Holter monitoring and exercise testing, which provide prognostic information. All patients should be offered instructions for prophylaxis against infective endocarditis and should be advised to avoid dehydration and strenuous exertion.13 15
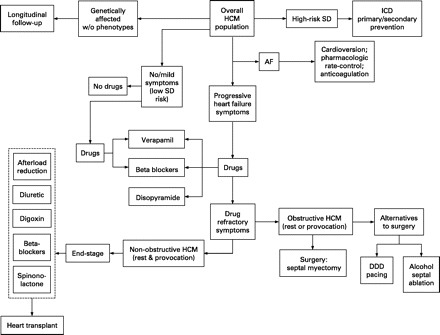
Symptoms such as exertional dyspnoea, orthopnoea, paroxysmal nocturnal dyspnoea and fatigue are common, characteristically in preserved LV contractility and independent of whether outflow obstruction is present.16 They appeared largely caused by diastolic dysfunction with impaired filling due to abnormal relaxation and increased chamber stiffness, leading in turn to elevated left atrial and LV end-diastolic pressures, pulmonary congestion and impaired exercise performance.17 These symptoms may also intertwined with other important pathophysiological mechanisms such as myocardial ischaemia, outflow obstruction associated with mitral regurgitation and atrial fibrillation. Because typical anginal chest pain may be part of the HCM symptom-complex, associated atherosclerotic CAD, which may complicate clinical course, it is indicated in patients with HCM and persistent angina who are over 40 years of age or who have risk factors for CAD, or when CAD is judged possible prior to any invasive treatment for HCM.4 The treatment strategy for a wide spectrum of clinical presentation of HCM can be seen in fig 1.
Clinical presentation and treatment strategies for patient subgroups within the broad clinical spectrum of hypertrophic cardiomyopathy (HCM). AF, atrial fibrillation; DDD, dual-chamber; ICD, implantable cardioverter-defibrillator; SD, sudden death. Reproduced from Maron et al.4
Beta-blockers
Propranolol was the first drug used in the medical management of HCM, and long-acting preparations of propranolol or agents such as atenolol, metoprolol or nadolol have been employed more recently. The beneficial effects of beta-blockers on symptoms of exertional dyspnoea and exercise intolerance appear to be attributable largely to a decrease in the heart rate with a consequent prolongation of diastole and relaxation, and an increase in passive ventricular filling. These agents lessen LV contractility and myocardial oxygen demand, and possibly reduce microvascular myocardial ischaemia. Substantial experience suggests that standard dosages of these drugs can mitigate disabling symptoms and limit the latent outflow gradient provoked during exercise when sympathetic tone is high and heart failure symptoms occur. However, there is little evidence that beta-blocking agents consistently reduce outflow obstruction under resting conditions. Consequently, beta-blockers are a preferred drug-treatment strategy for patients with symptoms with outflow gradients present only with exertion, and currently available data do not support the routine use of beta-blockers in the prevention of sudden cardiac death in these patients.4 18
Verapamil
Calcium antagonist verapamil has been widely used empirically in both the non-obstructive and obstructive forms, with a reported benefit for many patients, including those with a component of chest pain. It has been common practice, however, to administer verapamil to those patients who do not experience a benefit from beta-blockers or who have a history of asthma. Limited reports also suggested more benefit with the use of verapamil instead of beta-blocker.19 20 Nevertheless, verapamil has also been reported to cause more deaths in a few HCM patients with severe disabling symptoms and markedly elevated pulmonary arterial pressure in combination with marked outflow obstruction.19 21 At present, there is no evidence that combined medical therapy with administration of betablockers and verapamil is more advantageous than the use of either drug alone.4
Disopyramide
There are reports of disopyramide producing symptomatic benefit in severely limited patients with resting obstruction, because of a decrease in SAM, outflow obstruction and mitral regurgitant volume.22 Because disopyramide may cause accelerated atrioventricular (A-V) nodal conduction and thus increase ventricular rate during AF, supplemental therapy with beta-blockers in low doses to achieve normal resting heart rate has been advised. Although disopyramide incorporates antiarrhythmic properties, there is little evidence that proarrhythmic effects have intervened in HCM patients. Nevertheless, this issue remains of some concern in a disease associated with an arrhythmogenic LV substrate; prolongation of the QT interval should be monitored while administering the drug. Furthermore, disopyramide administration may be deleterious in non-obstructive HCM by decreasing cardiac output, causing most investigators to limit its use to patients with outflow obstruction who have not responded to beta-blockers or verapamil.4
Other pharmacological agents
At present, the information regarding drugs such as sotalol and other calcium antagonists (such as diltiazem) is insufficient to recommend their use in HCM. Diuretic agents may be added to the cardioactive drug regimen prudently, preferably in the absence of marked outflow obstruction. Because many patients have diastolic dysfunction and require relatively high filling pressures to achieve adequate ventricular filling, it may be advisable to administer diuretics cautiously. Nifedipine, because of its particularly potent vasodilating properties, may be deleterious, particularly for patients with outflow obstruction. Also, administration of nitroglycerine, ACE inhibitors or digitalis is generally contraindicated or discouraged in the presence of resting or provocable outflow obstruction.4 14 A prospective study done by Yamazaki et al indicates the efficacy of angiotensin II receptor blockers (ARB) to ameliorate myocardial impairment in hypertrophic nonobstructive cardiomyopathy by reducing left ventricular mass compared with placebo.23
Surgery
Although medical therapy improves symptoms in most patients, a subgroup will need further intervention. If the resting gradient is greater than 30 mm Hg (or the provocable gradient is greater than 50 mm Hg) and if the patient continues to have symptoms of dyspnoea or angina that limit daily activity, other invasive interventions may be considered.14 15 Symptomatic benefit following myectomy appears to be largely the consequence of abolishing or reducing the basal outflow gradient and mitral regurgitation, and restoring normal LV systolic and end-diastolic pressures, which may also favourably influence LV diastolic filling and myocardial ischaemia. Isolated myectomy is now performed with a low operative mortality in centres with extensive experience with the procedure, 1–3%, and even less.13 15 24 Surgical risk may be higher among very elderly patients (particularly those with severe disabling symptoms associated with pulmonary hypertension), patients with prior myectomy or those undergoing additional cardiac surgical procedures. Complications such as complete heart block (requiring permanent pacemaker) and iatrogenic ventricular septal perforation have become uncommon (⩽1–2%), while partial or complete left bundle-branch block is an inevitable consequence of the muscular resection and is not associated with adverse sequelae.4
Dual-chamber pacing
Several groups investigated the effects of permanent dual-chamber pacing on severe outflow obstruction and refractory symptoms within observational and uncontrolled study designs reporting dual-chamber pacing to be associated with a substantial decrease in outflow gradient, as well as amelioration of symptoms in most patients.25 A reduction of gradient with pacing in turn consistently relieved symptoms. However, other catheterisation laboratory studies showed that a decrease in the outflow gradient produced by temporary A-V sequential pacing could be associated with detrimental effects on ventricular filling and cardiac output. Two randomised, crossover, double-blind studies (one multicentre and one from the Mayo Clinic) reported the effects of pacing in HCM patients to be less favourable than the observational data had suggested.26 Objective measures of exercise capacity (eg, treadmill exercise time and maximum oxygen consumption) did not differ significantly during pacing and without pacing. These observations demonstrate that subjectively reported symptomatic benefit during pacing frequently occurs without objective evidence of improved exercise capacity. Furthermore, no correlation has been demonstrated for gradient reduction between short- and long-term pacing, suggesting that testing the gradient response to short-term pacing in the catheterisation laboratory has limited practical clinical value in judging long-term efficacy.26 However, the failure to achieve gradient reduction with temporary pacing suggests that permanent pacing is probably not indicated. The ACC/AHA/NASPE 2002 guidelines have designated pacing for severely symptomatic and medically refractory HCM patients with LV outflow obstruction as a class IIB indication.27
Percutaneous alcohol septal ablation
The more recently developed alcohol septal ablation technique, a catheter interventional treatment, involves the introduction of absolute alcohol into a target septal perforator branch of the left anterior descending coronary artery for the purpose of producing a myocardial infarction within the proximal ventricular septum. It mimics the haemodynamic consequences of myectomy by reducing the basal septal thickness and excursion (producing akinetic or hypokinetic septalmotion), enlarging the LV outflow tract and, thereby, lessening the SAM of the mitral valve and mitral regurgitation. Successful alcohol septal ablation may trigger a rapid reduction in resting outflow gradient evident in the catheterisation laboratory. More frequently, a progressive decrease in the gradient occurs after 6–12 months, usually achieving levels in a range equivalent to that with myectomy, and resulting from remodelling of the septum without significant impairment in global LV ejection The mortality and morbidity associated with alcohol ablation in experienced centres have proved to be relatively low, but the extent to which remodelling occurs with time secondary to this procedure is unpredictable and not fully understood. Also, there is concern that extensive wall thinning could lead to arrhythmogenic susceptibility or even the end-stage phase. Reports of permanent pacemaker implantation for induced high-grade A-V block have ranged from 5% to as high as 30%, but this complication appears to be decreasing substantially with the use of smaller amounts of alcohol. In contrast to septal myectomy, which usually produces left bundle branch block, alcohol ablation commonly results in right bundle branch block. Procedural complications may have occurred as a result of coronary artery dissection or backward extravasation of alcohol, producing occlusion or abrupt coronary no-flow, and a large anteroseptal myocardial infarction. Candidates for alcohol septal ablation should have severe heart failure symptoms (NYHA classes III or IV) refractory to all medications utilised in HCM as well as a subaortic gradient of 50 mm Hg or more measured with Doppler echocardiography either under basal conditions or with physiological provocative manoeuvres during exercise. Alcohol septal ablation potentially creates a permanent arrhythmogenic substrate in the form of a healed intramyocardial septal scar that could increase the risk of lethal re-entrant arrhythmias.
References
Footnotes
BBS and RA conceived and wrote the paper. BBS is the guarantor for this paper.
Competing interests None.