Article Text
Abstract
Atherosclerosis of the lower extremities frequently leads to lifestyle-restricting claudication and can cause critical limb ischaemia (rest pain, non-healing ulcer, or gangrene). The prevalence of peripheral arterial disease (PAD) is rising in line with an ageing population. In the USA, PAD affects 8–10 million people (approximately 12% of the adult population). There is a strong association with concomitant coronary artery and cerebral vascular disease in these patients, which represents a significant cause of mortality and morbidity in patients with PAD. Disease affecting the lower extremity peripheral vessels is most aggressive in smokers and diabetics.
Statistics from Altmetric.com
Atherosclerosis affecting the lower extremities frequently leads to lifestyle-limiting claudication and can lead to critical limb ischaemia (CLI; rest pain, non-healing ulcer, or gangrene). The prevalence of peripheral arterial disease (PAD) is increasing with an ageing population. In the USA, PAD affects 8–10 million people (approximately 12% of the adult population).1 Over the last decade, advancements in interventional techniques and technologies, along with patient preference, have shifted revascularisation strategies from traditional open surgical approaches toward lower morbidity percutaneous endovascular treatments. Endovascular therapy is performed with local anaesthesia, which enables the treatment of patients who are at high risk for general anaesthesia. Patients are ambulatory on the day of treatment and can often return to normal activity within 24–48 h of an uncomplicated procedure. In principle, endovascular therapies generally do not preclude or alter subsequent surgery and may be repeated if necessary.
This paper aims to review the current indications, outcomes and current endovascular modalities for the treatment of lower extremity peripheral arterial disease.
Indication for endovascular therapy
Intermittent claudication
Impairment of the arterial circulation to the leg may cause intermittent claudication (exertion-related discomfort that affects buttocks, thigh, or calf muscles and is relieved with rest).
Patients with lifestyle-interfering claudication who have aortoiliac disease should undergo endovascular therapy as the initial therapy, because patients with proximal disease do not respond as well to exercise and medical therapy. Those with disease below the inguinal ligament should undergo a trial (3 to 4 months) of medical therapy that consists of a walking programme and cilostazol which have been shown to increase walking distance by as much as 50% compared with baseline.2 3 Patients with claudication progress to limb loss at a rate of <5% per year. Therefore, revascularisation is reserved for those patients with favourable anatomy who fail conservative therapy and have vocation-limiting or lifestyle-limiting symptoms (table 1). The therapeutic goals include symptom relief, increased walking distance, and improved functionality and quality of life. For these reasons, durability of the procedure becomes important, because recurrent ischaemic symptoms require repeated procedures.
CLI
CLI is defined as rest pain, non-healing ulcer, gangrene or tissue loss that is caused by severe compromise of blood flow to the affected extremity. Nearly half of all patients with CLI will need revascularisation and among those who have disease that is not revascularable, approximately 40% will require major amputation.4 The incidence of major amputation is 5–10 times higher in diabetics. Additionally, many patients with CLI have increased comorbidities such as coronary or pulmonary disease resulting in increased risk of any intervention, especially open surgery. The clinical outcome for patients presenting with CLI is poor, with a 1-year death rate of 22%.5
Patients with CLI have significantly severe restrictions in arterial flow, often with multilevel disease and compromised outflows. Small increase in the level of blood flow may be adequate to relieve rest pain but more blood flow is required for healing ulcers or surgical incisions after distal bypass or amputation.
In patients with CLI, non-invasive parameters may be helpful in assessing the likelihood of wound healing. Ankle–brachial indices (ABIs) <0.5, ankle systolic pressure <50 mm Hg, toe systolic pressure <30 mm Hg and transcutaneous partial pressure of oxygen (TCpO2) <30–50 mm Hg are consistent with CLI and impaired wound healing potential. CT and MRI angiography are emerging non-invasive modalities in the evaluation of PAD and help greatly in the planning of complex procedures. The current generation of CT and MRI may not always provide specific information regarding the infrapopliteal anatomy in great detail, but often will provide excellent images of the iliofemoral inflow arteries.
Anatomy suitable for endovascular therapy is often present in one or more below-knee vessels. Therapy must be designed to restore pulsatile, straight-line flow to the distal limb. The principle is that less blood flow is required to maintain tissue integrity than to heal a wound, so restenosis does not always result in recurrent CLI unless there has been repeated injury to the limb. Therefore, the emphasis is less on long-term vessel patency and more on amputation-free survival.
Lesion selection and outcomes
Aortoiliac disease
Iliac or aortoiliac artery disease can manifests as hip, thigh, or leg claudication. Isolated stenosis or occlusion of the terminal aorta is uncommon but can result in the Leriche syndrome with claudication of the upper thighs and buttocks, erectile dysfunction and diminished femoral pulses. Obstructive atherosclerotic disease of the terminal aorta and the iliac arteries is preferentially treated with endovascular techniques rather than surgery. The use of stents can achieve long-term patency rates comparable to aortofemoral bypass surgery, even in complex lesions, and without the associated morbidity and mortality.6 A meta-analysis of 14 studies involving either balloon angioplasty or stenting to treat iliac stenoses revealed higher procedural success and a 39% lower risk of long-term failure with stenting compared with balloon angioplasty.7 The 3-year assisted patency rate for stenting of occluded iliac arteries is 80% to 90%6 8 compares favourably to the 5-year patency of 88% to 91% for aortobifemoral bypass grafts without the associated 8.3% surgical morbidity and 3.3% surgical mortality.9 Lower primary patency with endovascular revascularisation of iliac arteries has been noted in the presence of diabetes, CLI, poor distal runoff, renal insufficiency and female gender.10
Common femoral artery disease
Atherosclerotic disease affecting the common femoral artery is best treated surgically with an iliofemoral bypass operation or endarterectomy with patch angioplasty.11 As this vessel lies over the hip joint, placement of stents is suboptimal, and restenosis or stent thrombosis can be associated with acute limb-threatening ischaemia, as circulation to the superficial femoral artery (SFA) and the profunda femoris can be compromised simultaneously. Common femoral artery balloon angioplasty and debulking by atherectomy can be considered for patients who are poor candidates for surgery and present with CLI or severe lifestyle-limiting claudication.12 13
Superficial femoral and popliteal artery disease
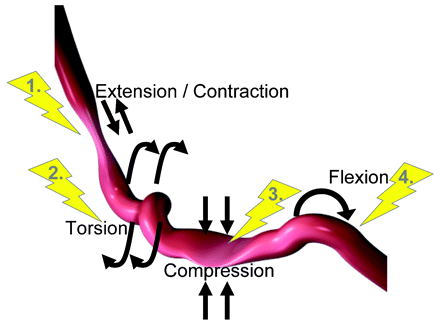
In general, percutaneous treatment of the SFA by balloon angioplasty or stenting is associated with suboptimal long-term clinical outcomes. The majority of lesions in the SFA are diffusely disease, calcified and associated with chronically occluded segments. Short, focal (<5 cm) lesions in the SFA which is uncommon, respond well to balloon angioplasty alone, with primary and secondary patency rates comparable to stenting.14 Endovascular stenting in the SFA is generally indicated in the presence of a flow-limiting dissection or severe elastic recoil after balloon angioplasty. For long lesions in the SFA (up to 10 cm) in length, endovascular stenting with nitinol self-expanding stents or atherectomy seem reasonable options and are superior to balloon angioplasty alone. However, vessel and stent flexion, elongation and torsion during routine daily activities are associated with the development of stent fractures and subsequent restenosis (fig 1).
Schematic showing different forces exerted along the superficial femoral artery.
In a recent meta-analysis involving 934 patients, the 1-year primary patency rates following balloon angioplasty ranged from 45% to 84.2% and at 2 years it varied from 25% to 77.2%. Following stent implantation, the 1-year primary patency rates varied from 63% to 90% and 2-year primary patency ranged from 46% to 87%.15 These 1-year patency results are comparable to surgical revascularisation.
Infrapopliteal disease
Percutaneous intervention for infrapopliteal disease is generally performed in patients presenting with CLI or for limb salvage. These patients differ from patients presenting with intermittent claudication in that dilatation of inflow stenoses alone is usually not enough. Instead, they require achievement of straight-line flow into the pedal arch to sustain viability of the limb and promote wound healing. The primary goal of intervention in patients with CLI is not long-term patency, but rather the avoidance of a major amputation. The vascular presentations of patients with infrapopliteal disease and CLI are diverse, including short, segmental stenoses and occlusions; diffuse, obstructive diseases; and long segments of total occlusion with, at times, obscure regions of recanalisation.
There are no randomised controlled trials or guidelines regarding endovascular management of infrapopliteal disease. The decision to intervene for infrapopliteal occlusive disease must be based on clinical grounds, as most patients with infrapopliteal disease are asymptomatic. Intermittent claudication due to isolated infrapopliteal disease is rare and can be treated medically. However, CLI due to isolated infrapopliteal disease often occurs in patients with diabetes and renal failure, where infrapopliteal vessels may be severely and diffusely involved but with relative sparing of the iliac and femoropopliteal vessels. CLI is of particular concern in diabetics, who represent a majority of the patients undergoing limb salvage procedures.
Although technically feasible, the evidence to support endovascular therapy as the primary approach for limb salvage in patients with CLI with infrapopliteal disease is still evolving. In a recent study of 443 below-the-knee interventions for CLI, the primary patency and limb salvage rates were 74% and 97%, respectively.16 These patency and limb salvage rates compare favourably with published surgical data.
Endovascular therapy
The traditional endovascular approach includes the use of balloon angioplasty and/or balloon-expandable or self-expanding bare metal stents. The effectiveness of balloon angioplasty and stent placement depends on the complexity of the underlying disease. Treatment of short and focal lesions has the best outcomes. While technical success and short-term and medium-term patency rates achieved with these traditional modalities have been promising, long-term patency rates, especially in the treatment of femoropopliteal disease, have proven disappointing. Vessel elastic recoil and high rates of flow-limiting dissections remain major limitations of balloon angioplasty. The limitations of conventional balloon angioplasty have led to increased use of stents and adjunctive endovascular modalities to improve patient outcomes (table 2).
Adjunctive endovascular modalities
Balloons and stents
Cryoplasty
Cryoplasty is a technique that combines cold therapy and balloon angioplasty. Cooling of the balloon is achieved by the use of liquid nitrous oxide as the balloon inflation media. Potential beneficial effects of cooling include plaque modification, reduction of elastic recoil and induction of apoptosis in the smooth muscle cells in the vessel wall and hence reduced restenosis. Several clinical trials have demonstrated the safety and efficacy of PolarCath (Boston Scientific, Natick, Massachusetts, USA) in patients with SFA and patients with CLI with infrapopliteal disease.17 18 Immediate success rates were high but long-term patency rates of 75% at 3 years were similar to the results of conventional balloon angioplasty.
Self-expanding nitinol stents
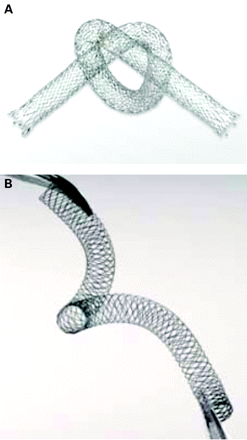
Self-expanding stents are ideal for lesions in the SFA which are subjected to major torsion, flexion and extension forces across the hip and knee joints and within the muscular portion (adductor canal) of the thigh that result in stent fracture in up to 25% of cases.19 Nitinol is an alloy characterised by its super elasticity (it returns to its original shape when an external force is removed) and thermal shape memory (it returns to a preformed shape on warming, allowing self-expansion) which make these stents resistant to compression and deformation (fig 2).
Examples of newer generation nitinol self-expanding stents ((A) LifeStent (Bard Peripheral, Tempe, Arizona, USA) and (B) Supera (IDEV Technologies, Houston, Texas, USA)) designed to be more flexible and resistant to fracture for use in the superficial femoral artery.
In the study by Schillinger et al, 104 patients with CLI and SFA stenosis were assigned to primary nitinol self-expanding stent implantation or angioplasty. At 2 years, primary stenting was associated with a significant reduction in restenosis (45.7% vs 69.2%, p=0.03) and a trend toward clinical benefit compared with balloon angioplasty with optional stenting.20 21 However, in a recent meta-analysis of 10 randomised trials comparing primary stenting and angioplasty with provisional stenting with a follow-up period of 9 to 24 months, despite higher immediate success, routine nitinol stenting was only associated with a trend towards reduction in target vessel revascularisation (relative risk (RR)=0.79, 95% CI=0.59 to 1.06, p=0.12).22
Drug eluting stents
The success of drug-eluting stents (DES) in the suppression of neointimal hyperplasia and the reduction of restenosis in the coronary arteries has led to enthusiasm in their use in the treatment of PAD. Two trials (SIROCCO 1 and 2; for “Sirolimus-Coated Cordis Self-expandable Stent”) evaluated the efficacy of a sirolimus-coated nitinol self-expanding stent compared to a bare metal stent in the treatment of occlusive SFA disease.23 24 The efficacy of DES shown in the coronary arteries does not seem to be replicated in the peripheral arteries. At 2 years, the restenosis rate of DES remained no different to BMS (22.9% vs 21.1%) in the combined SIROCCO 1 and 2 cohorts.25 A recent randomised trial demonstrated that a paclitaxel-coated balloon catheter (Paccocath, Bavaria Medizin Technologie, Oberpfaffenhofen, Germany) was effective in reducing the 6-month restenosis when used in the SFA.26 Stent-based or balloon-based drug elution in the periphery is an exciting prospect. However, many issues remain to be resolved, including the ideal pharmacological agent and release kinetics.
Drug-eluting stents appear more promising in the treatment of infrapopliteal disease. Marked reduction in restenosis at 6 months from 55% in bare metal stents to 4% (p<0.001) in the DES was shown in one study.27 Although these “coronary-like” results are encouraging, longer follow-up are required, especially concerning late thrombosis risk as seen in the coronary application of DES.
Stent grafts
Stent grafts have been developed in an effort to replicate the surgical gold standard of femoropopliteal bypass. The Viabahn endoprosthesis (WL Gore & Associates, Flagstaff, California, USA) is a self-expanding nitinol stent mounted to the outside surface of a tube of expanded polytetrafluoroethylene (PTFE), which acts as a barrier to neointimal formation (fig 3). A randomised study comparing the treatment of SFA occlusive disease with the Viabahn stent and prosthetic femoral to above-knee popliteal artery bypass graft showed comparable primary patency rates at 12 months (73.5% vs 74.2%, respectively).28 Primary and secondary patency rates 55% and 80% have been demonstrated up to 4 years for long SFA lesions treated with the Viabahn stent graft.29 However, major disadvantages of stent grafts include low radial strength and increased risk of thrombosis in up to 10%.30
Diffuse in-stent restenosis (arrows) in the (A) proximal and (B) distal superficial femoral artery (SFA). Implantation of the Viabahn Endoprosthesis (Gore & Associates) (C) from the proximal (D) to distal (E) SFA.
Debulking techniques
Excimer laser atherectomy
The 308-nm excimer laser catheter (Spectranetics, Colorado Springs, Colorado, USA) delivers bursts of ultraviolet energy in short pulse durations and removes a tissue layer of 10 µm by a photochemical rather than thermal process.31 Potential advantages of laser atherectomy include the ability to ablate thrombus and to inhibit platelet aggregation and for treating long occlusions and complex disease. However, results for excimer laser atherectomy have been modest. The Peripheral Excimer Laser Angioplasty (PELA) trial showed high technical success rates but no difference in clinical events or patency rates at 1 year of follow-up.32 However, use of the excimer laser did result in less distal embolisation and a trend towards a lower stent implantation rate compared to angioplasty alone. In the Laser Angioplasty for Critical Limb Ischaemia (LACI) trial, 145 patients with CLI who were not surgical bypass candidates were treated with excimer laser-assisted intervention achieved excellent limb salvage rate of 93% at 6 months.33 Excimer laser may be a useful adjunctive debulking tool where angioplasty results are poor.
Excisional atherectomy
The SilverHawk Plaque Excision System (ev3 Endovascular, Plymouth, Minnesota, USA) is a forward-cutting atherectomy device. When the catheter is advanced through a lesion, a high-speed cutting blade excises a ribbon of plaque that is collected into the catheter nose cone. Multiple catheter passes are made through the lesion, during which the blade is redirected sequentially toward all quadrants of the vessel lumen. Zeller and colleagues treated 45 de novo, 43 restenotic and 43 in-stent restenotic femoropopliteal lesions with 86% technical success after atherectomy alone and 100% after adjunctive therapies. Primary patency was 73%, 42% and 49% in the three groups, respectively, at 18 months. The authors concluded that long-term technical and clinical results after atherectomy of femoropopliteal lesions are in favour of de novo lesions compared with restenotic lesions.34
Rotational atherectomy
The Pathway Medical PV (Pathway Medical Technologies, Redmond, Washington, USA) system has expandable, rotating scraping blades (“flutes”) with ports between the flutes that allow flushing and aspiration of plaque material or thrombus. The Orbital Atherectomy System (Cardiovascular Systems, St Paul, Minnesota, USA) is a high-speed rotational atherectomy system that incorporates an eccentric, diamond-coated abrasive crown. When rotated at high speeds, the abrasive crown moves in an orbital path within the artery, potentially creating a lumen larger than the diameter of the crown while generating only small particles that will pass through the capillary circulation
Subintimal angioplasty
The technique of subintimal angioplasty was first described by Bolia et al35 for treatment of long occlusions that do not respond to conventional guidewire and angioplasty. During this procedure, a guidewire is intentionally directed into the subintima to create a dissection plane that is then extended distally beyond the occlusion where upon the wire is redirected back into the true lumen (fig 4). Using this technique, complex lesions including long occlusions (>15 cm), highly calcified occlusions and diffuse tandem lesions can be effectively crossed with the guidewire and then treated using conventional methods. The 1-year patency rates for subintimal angioplasty range from 53% to 92%.36 37 Unfortunately, subintimal angioplasty is limited by technical failure up to 25% due to inability to re-enter the true lumen from the subintimal space.38
(A) Long (>10 cm) calcified total occlusion in the superficial femoral artery (SFA). (B) Subintimal angioplasty technique using a 0.089 mm guidewire looped upon itself and advanced beyond the occlusion in the subintimal space (arrow). (C) Re-entry of the guidewire back into the true lumen (arrow) using the OutBack LTD re-entry catheter (Cordis), which has a 22-gauge re-entry needle at the distal end. The device is aligned with the true lumen at the desired re-entry location where the needle and guidewire are advanced into the true lumen (arrow). (D) Final result after balloon angioplasty and implantation of self-expanding nitinol stent in the SFA. (E) Picture of the OutBack LTD Re-entry catheter (Cordis).
Total occlusion crossing devices
The CROSSER chronic total occlusion recanalisation system (FlowCardia Inc, Sunnyvale, California, USA) is a device that uses high-frequency mechanical vibrations (20 000 cycles/s to a depth of 20 µm) propagated to the wire tip. The vibrational mechanical impact and cavitational effects result in penetration of the occluded artery. The CROSSER system has previously been studied in chronic total occlusions in the coronary circulation with high technical success rates (63% to 76%).39
The FrontRunner XP chronic total occlusion catheter (Cordis, J&J, Miami, Florida, USA) performs controlled blunt microdissection using a pair of miniature hinged jaws. Controlled blunt microdissection has been reported to be safe and feasible, with a 91% success rate in treating iliac and lower limb chronic total occlusions in a series of 44 chronic total occlusions that had failed conventional percutaneous revascularisation.40
Total occlusion re-entry devices
The OutBack LTD re-entry catheter (Cordis) is a catheter with a 22-gauge re-entry needle at the distal end. The device is passed over a guidewire into the subintimal space adjacent to the desired re-entry location. Orienting the catheter under fluoroscopy aligns the true lumen with the needle, which is then advanced into the true lumen. The wire can then be advanced into the true lumen, and conventional therapies can be delivered (fig 4). In a series of 100 total occlusions which could not be re-entered, the reported clinical success rate in re-entering the true lumen with the re-entry catheters and successfully completing the intervention was 95%.41
The Pioneer catheter (Medtronic, Minneapolis, Minnesota, USA) incorporates a distal 25-gauge re-entry needle with an integrated intravascular ultrasound transducer to allow directed ultrasound-guided re-entry into the true lumen. Likewise, the device is brought into the subintimal tract over a wire, and under intravascular ultrasound imaging, colour flow is identified in the true lumen into which the needle is advanced and the true lumen is wired.42
Surveillance post intervention
One strategy for follow-up would be to obtain a post-treatment ABI early after the procedure to establish a new baseline. Patients return for a follow-up clinical examination and ABI between 3 and 6 months after the procedure to assess continued patency and symptom relief. An imaging study to determine patency (duplex ultrasound or CT angiography) of the treated lesion is performed if there is suspicion of restenosis (ie, return of symptoms or a significant (>0.15) fall in the ABI). Routine duplex ultrasound surveillance after lower-extremity endovascular procedures is not recommended. Annual follow-up with a goal toward continued risk factor management should include ABI determinations and interval histories.
Conclusions
Over the past decade, the safety and efficacy of endovascular interventions for PAD have improved greatly due to advancements in catheter-based techniques and technologies. Procedural outcomes and late primary patency rates after endovascular therapies are improving and often comparable to those achieved with surgical revascularisation. It is now possible to treat lesions through endovascular therapy that would have been treated surgically in the past. The goals of endovascular treatment are to improve vascular patency and limb perfusion, to eliminate claudication and to optimise limb salvage for patients with CLI. Traditional interventions of balloon angioplasty with or without stent placement can achieve these results in many situations, while adjunctive or preferred use of the newer endovascular modalities can extend the reach and effectiveness of revascularisation with improved patient quality of life and avoidance of limb amputation in many more cases. In the future, it is anticipated that additional clinical experience and randomised trials will provide insights regarding selection of the optimal devices for specific anatomical subsets.
References
Footnotes
Competing interests None declared.
Provenance and Peer review Not commissioned; externally peer reviewed.