Article Text
Abstract
Background Multiple factors, in addition to left ventricular ejection fraction (LVEF) influence the risk of mortality in coronary artery disease. The purpose of this study was to evaluate the main causes of syncope after myocardial infarction (MI) and to propose an algorithm of management.
Methods 356 patients consecutively admitted for syncope and history of MI (>1 month), without ventricular tachycardia (VT), underwent echocardiography, Holter monitoring, head-up tilt test, exercise testing, signal-averaged ECG, electrophysiological study (EPS) and evaluation of coronary status. The mean follow-up was 4±2 years.
Results Monomorphic VT, ventricular flutter or fibrillation (VF) and supraventricular tachyarrhythmia were respectively induced at EPS in 87, 63 and 39 patients; conduction disturbances were noted in 23 patients, and 57 patients had several abnormalities. Among the 144 patients with negative EPS, coronary ischaemia was identified in 37 patients, and hypervagotonia in 27 patients. All studies remain negative in 84 patients (23.6%), more frequently women (p<0.001). Four patients died suddenly during follow-up. A longer QRS duration, a lower LVEF and grade IVa,b of Lown on Holter ECG were associated with the induction of VT. LVEF<40% and VT/VF induction were predictors of cardiac mortality, VT was a predictor of sudden death, and low LVEF and advanced age were predictors of death by heart failure.
Conclusion Myocardial ischaemia, hypervagotonia, conduction abnormalities, ventricular or supraventricular tachyarrhythmias were identified in 76% of patients with syncope after MI. Several factors of syncope were found in 57 patients (16%). Non-invasive rhythmological and systematic coronary status assessment should be recommended in patients with syncope following MI.
- Syncope
- myocardial infarction
- electrophysiological study
- prognosis
- management
Statistics from Altmetric.com
Patients with syncope and history of myocardial infarction are at risk of sudden death. In the era of implantable cardioverter defibrillator (ICD),1 the left ventricular ejection fraction (LVEF) is probably a more important factor of mortality than syncope. However, causes for syncope following MI are multiple,2 and ICD implantation does not resolve all causes of syncope and cardiac death.3 Electrophysiological study (EPS) was widely used several years ago,4 5 but was considered without interest for the risk stratification in coronary heart disease.6 However, inducible VT remains an important and independent factor of cardiac mortality.7
The assessment of patients who present with a syncope following MI may be debatable. In some cases, it is limited to the evaluation of LVEF, and ICD is indicated if LVEF is lower than 36%.1 However, the proarrhythmic effect of ICD and other complications are well known,8 and for others this justifies a specific search of the cause of syncope with an adapted therapy. Among patients with LVEF higher than 35%, the implantation of a loop recorder is indicated9–11 until the recurrence of syncope with a risk of trauma or sudden death, if syncope recurs.
The purpose of the study was to evaluate the factors responsible for syncope and the risk of recurrence after a treatment guided by this evaluation. We then constructed an algorithm to manage the patients with syncope and history of myocardial infarction.
Population of study
All patients with a history of myocardial infarction, who presented with at least one episode of unexplained syncope, admitted for electrophysiological study were systematically recruited over a 15-year period. Unexplained syncope was defined as a short loss of consciousness, without any obvious aetiology. Obvious aetiology included paroxysmal second- or third-degree AV block, sustained supraventricular or ventricular arrhythmia, and vasovagal syncope induced by cough, miction or abdominal pain.5 Patients were excluded if they had unstable angina, recent acute myocardial infarction (<1 month), recent coronary angioplasty or coronary bypasses surgery (<6 weeks), if they were in NYHA III–IV stage, had end-stage non-cardiac disease or received chronic amiodarone treatment. Patients with drug-induced bradycardia or tachycardia (accounting for around 10% of syncope) were also excluded; they represent about 10% of admissions for syncope. Patients with effort-related syncope and obviously ischaemic syncope were excluded, because an electrophysiological study was not performed in these patients.
During the period of study, 371 patients were recruited, of whom 15 were excluded for drug-related syncope and obviously ischaemic-related syncope. Three additional patients were excluded because they were lost on follow-up.
The remaining 356 patients, aged 31 to 85 years (mean age 66±11; 56 women and 300 men), represent the study population.
Methods
Patients underwent several investigations in the absence of antiarrhythmic drugs after giving informed consent. Personal and familial clinical history, list of drugs taken at the time of syncope and clinical examination were initially noted. Most patients received ACE inhibitors. Beta-blockers and digoxin were stopped before EPS.
The following non-invasive studies were performed: (1) surface ECG; (2) 24 h Holter monitoring (Elatec); (3) thallium exercise scintigraphy or exercise testing in 240 patients able to perform the exercise; (4) LVEF at the time of investigations for syncope determined by 2D echocardiography and/or left ventricular angiography; (5) measurement of QRS duration at signal-averaged ECG; and (6) head-up tilt test without provocative drugs in 110 patients with negative EPS or inducible ventricular flutter or fibrillation.
The following invasive studies were performed: (1) right and left angiography and coronary angiography was indicated in 301 patients; (2) complete EPS according to a protocol previously reported12 was systematic. The protocol included assessment of sinoatrial conduction function and atrioventricular conduction. Programmed atrial stimulation was performed with up to two extrastimuli and then programmed right ventricular stimulation up to three extrastimuli at the right ventricular apex and right ventricular outflow tract. Short coupling intervals (<200 ms) were not used in our study. If the study remained negative, the protocol was repeated after 2 to 4 μg/min isoproterenol infusion. Arterial blood pressure was continuously monitored by an external sphygmomanometer (Baxter, Hayashikomaki, Japan). A carotid sinus massage was performed in the supine position, except in patients with a known carotid atheroma.
Definitions
Inducible supraventricular tachyarrhythmia was assumed to be related to syncope if it was sustained, that is, lasting at least 3 min, either spontaneously terminating but reproducible or permanent, associated with a decline in systolic arterial blood pressure of at least 30% and with symptoms similar to spontaneous dizziness.
Induced ventricular tachyarrhythmias were categorised as monomorphic VT (<270 bpm), ventricular flutter (>270 bpm) or ventricular fibrillation (VF).
Abnormal electrophysiological findings were categorised as sinus node dysfunction, conduction disturbances, hypervagotonia, inducible supraventricular tachyarrhythmia or inducible ventricular tachyarrhythmia (VT/VF) according to classical diagnostic criteria.12 When several anomalies were noted, including the induction of a ventricular tachyarrhythmia, the presumed cause for syncope was categorised in ventricular tachyarrhythmia.
Follow-up
Patients were followed from 1 to 8 years or until heart transplantation (mean 4±2).
A pacemaker was implanted in patients in whom conduction anomalies were noted at EPS. Patients with induced supraventricular or ventricular tachycardia were treated with an association of amiodarone and beta-blocker. The treatment of ventricular arrhythmias was electrophysiologically guided when LVEF was more than 35%, mainly before 2000. ICD was usually implanted in patients with still a rapid inducible VT or when LVEF was less than 36%. A specific treatment (percutaneous coronary angioplasty or coronary artery bypass surgery) was indicated in patients with ischaemia. The treatment of ischaemia was also indicated in patients with ischaemia and inducible arrhythmia.
Total cardiac mortality included deaths related to heart failure and sudden deaths. Sudden death was defined as an unexpected death from a cardiac cause within a short time period (>1 h); deaths in relation to the development of a spontaneous sustained VT were classified with sudden deaths. Most deaths occurred in our hospital; for those who died at home or in another hospital, we contacted the last present medical doctor and the family to classify the nature of death.
Statistical analysis
Quantitative data were expressed as mean±SD. Comparisons of patients according to the possible cause of syncope and then according to the follow-up were performed with the Student unpaired t test for quantitative data, with the χ2 test for discrete variables. A p value of <0.05 was considered as significant. Univariate analysis by the Cox method and multivariate analysis were performed to identify the independent variables predictive of cardiac and sudden death. Survival curves were calculated using the Kaplan–Meier product-limit method and compared using the logrank test. The predictive negative and positive values of programmed ventricular stimulation data to predict cardiac death were calculated.
Results
Non-invasive studies
The mean LVEF was 43±14%. Holter monitoring was abnormal with ventricular couplets or non-sustained VT in 141 patients (40%).
The head-up tilt test reproduced syncope in 23 patients who had negative EPS, in four patients in whom a ventricular flutter was induced and in eight with inducible atrial tachyarrhythmias. In other patients, the test remained negative or induced a small decrease in arterial blood pressure without any symptoms. Hypervagotonia was retained as the main diagnosis only if other investigations were negative.
Exercise testing was positive in 54 patients, and non-sustained polymorphic VT developed during exercise in 30 of them. Myocardial ischaemia (>40%) was noted during the thallium-201 exercise scintigraphy and was found to be the only abnormality in 37 patients. Coronary ischaemia was noted in 26 other patients, associated with inducible atrial tachycardia in eight, with inducible VT in 17 and with conduction abnormalities in one patient.
Electrophysiological study
Sustained monomorphic VT<270 bpm (from 150 to 265 bpm, mean 205 bpm) or syncopal non-sustained VT (frequency from 220 to 240 bpm) was induced in 87 patients (24%), more frequently in patients with low LVEF (37%) than in those with preserved LVEF (17%) (p<0.001). VF was induced in 63 patients (18%) with the same frequency in patients with low LVEF (19%) than in those with preserved LVEF (16%). Ventricular tachyarrhythmia was induced in a control state in 140 patients and after isoproterenol infusion in 10 patients. VT/VF was induced by one extrastimulus in 19 patients, two extrastimuli in 70 patients and three extrastimuli in 61 patients. Seventeen patients had associated coronary ischaemia.
Atrial tachyarrhythmia was the only finding in 39 patients (11%) (12 paroxysmal junctional tachycardia, 14 atrial tachycardia or flutter, 13 atrial fibrillation). The incidence was similar in patients with low LVEF (8.5%) and preserved LVEF (13%). The induction of atrial tachyarrhythmias was noted in association with another abnormality in 13 patients.
Conduction abnormality was the only finding in 23 patients (6%), seven of these with low LVEF and 16 with preserved LVEF. They were noted in association with VT/VF in 10 other patients, with atrial arrhythmias in five patients and hypervagotonia in three patients.
Carotid sinus massage was positive in 15 patients, but other arrhythmias were noted in these patients, and hypervagotonia was not retained as the cause of syncope.
EPS remained negative in 144 patients, with the same frequency in patients with low LVEF and in those with preserved LVEF.
Possible causes for syncope
Table 1 summarises the possible causes for syncope.
Main possible causes of syncope
Analysis of investigations
Non-invasive and invasive investigations were negative in 84 patients (23.5%). Three patients had non-cardiac-related syncope (table 2).
Presumed causes of syncope
Statistical analysis indicated that patients with conduction abnormalities were older than other patients (p<0.05), and patients with inducible ventricular tachyarrhythmia were more often men (p<0.01).
Table 3 reports the data on non-invasive studies according to the results of the electrophysiological study. Salvos of ventricular beats on Holter monitoring were more frequent in patients with inducible VT than in those without VT/VF. LVEF was lower in patients with inducible VT and VF than in those with negative programmed ventricular stimulation. Patients with inducible VT had a longer QRS duration than patients with inducible VF or negative programmed ventricular stimulation.
Clinical and electrophysiological data according to the induction of a ventricular tachyarrhythmia
Patients with negative studies were more frequently women than patients with inducible ventricular tachyarrhythmia. Table 4 reports the diagnosis value of programmed ventricular stimulation for the identification of a VT as cause for syncope.
Diagnostic value of programmed ventricular stimulation
Follow-up
(1 to 6 years, mean 4±2); 4 patients were lost of view after at least 2 years of follow-up. Patients were followed from 1 to 6 year (mean 4 ±2) Fifty-six patients died from a cardiac death; 19 of them died suddenly, and 37 died from heart failure (table 5). Four other patients died from a non-cardiac cause. Patients who died from heart failure were older than remaining patients. Cardiac defibrillator was implanted in 22 patients. When the patient had multiple positive tests, we attempted to treat each of them.
Clinical and electrophysiological data of total population according to the follow-up
Global survival was 65% at 6 years in patients with low LVEF and 80% in those with preserved LVEF (logrank 18.71; p<0.0004). Non sustained VTs on Holter monitoring were more frequent in patients who died suddenly (table 4). There was a higher total cardiac mortality in patients with inducible VT and VF than in those with negative electrophysiological study (p<0.001). Induced VT increased the risk of death from heart failure (p<0.01) and sudden death (p<0.001). Cox analysis indicated that inducible VT was more frequent in patients who died from cardiac death (p<0.001), from heart failure (p<0.02) or from sudden death (or who died suddenly) (p<0.01); low LVEF was another predictive factor of total cardiac death (p<0.0005), death by heart failure (p<0.01) or sudden death (p<0.01). Multivariate analysis indicated that induction of VT/VF and LVEF less than 40% are predictors of total cardiac mortality (OR respectively 10.2, 7.9); only VT induction was a significant predictor of SD (OR 6.9); advanced age and LVEF<40% were significant predictors of death by heart failure (OR 6.3, 5.5) (table 6).
Stepwise logistic regression analysis to identify the independent variables predictive of cardiac death, sudden death and death by heart failure
Only two patients with negative non-invasive and invasive studies had recurrences of syncope 18 months and 3 years respectively after the first event. Another complete evaluation including loop recorder implantation remained negative. Loop recorder implantation indicated in 8 other patients with negative studies did not reveal arrhythmias in 7 of them. Another one patient presented a non sustained VT but remained asymptomatic. However, no patient had syncope during recording.
Proposed algorithm to manage patients with syncope and history of myocardial infarction
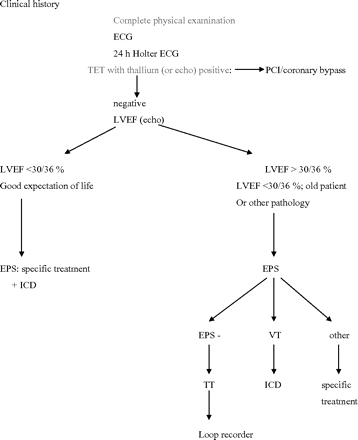
The management depends on several factors as the presence of other organic disease, the measurement of LVEF in stable conditions and the results of all investigations including systematic coronary status evaluation (figure 1).
Proposed diagram for the management of patients with syncope and history of myocardial infarction. –, negative; EPS, electrophysiological study; ICD, implantable cardiac defibrillator; LVEF, left ventricular ejection fraction; Other, abnomaly of electrophysiological study except inducible ventricular tachycardia; PCI, percutaneous coronary intervention; TET, Treadmill exercise testing; TT, tilt test; VT, inducible ventricular tachycardia.
Discussion
Non-invasive and invasive studies were abnormal in 76% of patients with syncope and a history of myocardial infarction. In patients with a negative evaluation, the risk of death was low and the recurrence of syncope rare. The study confirms the interest of exercise testing (associated with thallium scintigraphy or dobutamine echocardiography) and head-up tilt testing and the diagnostic value of EPS in postmyocardial infarction12 and in patients with syncope.3 13 14 Coronary ischaemia was not rare either as the sole factor of syncope or as in association with an arrhythmia. The high mortality in patients with low LVEF and inducible VT was previously reported.7 12 15–17 However, programmed ventricular stimulation does not predict sudden death but only ventricular occurrence of arrhythmias.2 12
The significance of inducible VF remains debated; the induction is considered without clinical significance in asymptomatic patients in postmyocardial infarction15 18 and in patients with syncope and coronary artery disease; in other studies, the VF induction is considered as pathological, but these studies included patients with spontaneous VT. In our study, we note a slightly higher cardiac mortality in patients with decreased LVEF and inducible VF than in patients with a negative study, but deaths were related to heart failure.
Negative EPS in patients with an LVEF less than 40% indicates a prognosis similar to a population without syncope15 19 and a favourable medium-term outcome, principally dependent on the value of LVEF.
Syncope in patients with heart disease has as many possible causes as in patients without heart disease. Bradyarrhythmias are not rare.20 Vagal hypertonia could be a frequent cause of syncope.21 Supraventricular tachyarrhythmia is reported in about 10% of the patients with syncope and heart disease.20 22 This last cause could be a frequent cause of syncope but remains difficult to prove: salvos of atrial premature beats on Holter monitoring are frequent and not specific. In patients with low LVEF, rapid supraventricular tachyarrhythmia induces a decrease in cardiac output. In patients with preserved LVEF, the increase in vagal tone in answer to the tachycardia could explain syncope, when tachycardia stops.22 Similar mechanism could explain why a ventricular monomorphic VT can induce syncope when LVEF is preserved; previous studies showed that a VT or a rapid ventricular pacing induces an adrenergic tone increase followed by a secondary vagal hypertonia.
Another facilitating mechanism for syncope associated with supraventricular or ventricular tachycardia was the presence of coronary ischaemia.23 Syncope is usually exercise-related and might be related to an ischaemic non-sustained polymorphic VT and to a decrease in cardiac output when a narrowing of main left coronary artery is present. Several studies have shown that anti-ischaemic treatment did not prevent the recurrences of tachycardia but improved their tolerance.23 24 Moreover, ischaemia is an independent predictor of death, and Elhendy et al23 reported that the combination of ischaemia and a positive EPS is associated with a very high rate of events. Shaw et al25 recommended an aggressive drug evaluation by serial myocardial perfusion single photon emission computer tomography to enhance the prognosis.25 Therefore, we recommend a systematic evaluation of the coronary status in the case of unexplained syncope occurrence in patients with a history of coronary heart disease, even in the absence of symptoms such as exercise-related syncope or syncope associated with chest pain. Myocardial ischaemia was noted in 15% of our population, and in 10% of this population, ischaemia was the only cause for syncope. The treatment by coronary angioplasty or coronary bypass surgery of coronary narrowing suppressed the recurrence of syncope.
Limitations of the study
The role of myocardial ischaemia alone or associated with a tachyarrhythmia could have been underestimated in the present study, because thallium exercise scintigraphy was not systematic, and only an exercise test was performed in 240 patients.
The incidence of neurocardiogenic syncope may also have been underestimated because the tilt test was not systematic. A patient may have supraventricular or ventricular tachycardia and also enhanced vagal tone.
The changes in treatments during the 15 years of recruitment could have modified the prognosis of our patients. However, a previous study published in 200326 reported the data of patients recruited earlier, and the prognosis was similar.
EPS was performed in a supine position, and only patients with a rapid non-sustained monomorphic VT reproduced their syncope. In those with inducible supraventricular tachycardia, only dizziness or a drop in arterial blood pressure was noted.
Data from the loop recorder implantation were not reported because the system was implanted in only eight patients with negative EPS.
The exact value of LVEF considered as pathological has changed during the recruitment, but we know also that values between 30 and 40% are frequently variable, and the indications of ICD varied from 30 to 40%, depending on the NYHA stage, the presence of NS VT at Holter monitoring and several other factors.
In conclusion, several causes such as coronary ischaemia, neurocardiogenic origin or supraventricular tachyarrhythmia might be implicated in the mechanism of syncope after myocardial infarction. The evaluation of coronary status is recommended; silent myocardial ischaemia was probably an underestimated facilitating cause of syncope. Thus, complete EPS remains a rapid tool to identify atrioventricular block, supraventricular or ventricular tachyarrhythmia. Induced monomorphic VT was the best predictor of sudden death. Advanced age and low LVEF were predictors of death by heart failure. Patients with unexplained syncope were at low risk of event.
References
Footnotes
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.