Article Text
Abstract
Coronary artery bypass grafting (CABG) remains one of the most common surgical procedures. In spite of great advancements like arterial grafts and off-pump bypass procedure, recurrent ischaemia may ensue with the lesions of the graft. Early postoperative ischaemia (<30 days) is due to graft occlusion or stenosis, and percutaneous coronary intervention (PCI) is frequently feasible. Late postoperative ischaemia (>3 years) is most often due to a saphenous vein graft (SVG) lesion. Multiple diseased grafts, reduced left ventricular function, and available arterial conduits favour repeat CABG, whereas, a patent left internal mammary artery to left anterior descending favours PCI. Embolic protection reduces atheroembolic myocardial infarction during PCI of SVG and should be routinely used in treatment of SVG lesions. A variety of vasodilators may reduce the risk of or mitigate the consequences of no-reflow. Drug-eluting stents reduce restenosis in SVG grafts, and have become the default strategy for many interventionalists.
- Coronary Artery Disease
Statistics from Altmetric.com
Introduction
Coronary artery bypass grafting (CABG) is one of the most common surgical procedures the efficacy of which has been enhanced by the use of arterial grafts, off-pump procedure, and minimally invasive surgical techniques.1 Severe myocardial ischaemia appears in 3–5% of patients immediately after surgery. Thereafter, recurrent ischaemic syndromes occur in 4–8% of post-CABG patients annually.1 Patients experiencing recurrence of ischaemia after CABG have lesions in various anatomic distributions like saphenous vein graft (SVG), native arteries, internal mammary (IMA), radial, gastroepiploic graft, or proximal subclavian artery. The results of percutaneous coronary intervention (PCI) depend on the types of conduits (native artery, arterial, or SVGs), or the locations on the conduits (proximal, mid, distal, or at the anastomotic sites), and age of the grafts.2
Indications for intervention
The status of the left anterior descending artery (LAD) and its graft significantly influences revascularisation choices because of its impact on long-term outcome and lack of survival benefit of redo-CABG to treat non-LAD ischaemia.1 ,3 PCI is indicated in post-CABG symptomatic patients not suitable for redo surgery because of contraindications (pulmonary and renal failure, old age, malignancy). Other patients who can undergo PCI are patients with patent arterial grafts, relatively small amount of ischaemic myocardium, and patients with no arterial or venous conduit available for graft. Factors favouring redo-CABG include multivessel disease, severe vein graft disease, damaged ventricle and availability of arterial conduits.1
Early postoperative period
The most common cause of ischaemia within hours or days of surgery is acute vein graft thrombosis (60%). Other causes are incomplete surgical revascularisation (10%), kinked grafts, stenosis at the proximal or distal anastomotic sites, focal stenosis distal to the insertion site, inaccessible intramyocardial position of a recipient artery, or bypass of wrong vessel.2 ,4 Patients undergoing minimally invasive and off-pump techniques, and those receiving non-IMA grafts, have high risk of early postoperative ischaemia.5 Coronary angiography has been performed in some centres to determine the cause of early postoperative myocardial ischaemia.6–8Utmost care is essential to maintain intracoronary position of guidewire, conservative balloon sizing to avoid suture-line disruption and severe haemorrhagic complications. Immediate access to a covered stent is warranted should suture-line perforation occur. Recurrent ischaemia between 1 month and 1 year after CABG is mostly due to peri-anastomotic stenosis, graft occlusion, or mid-SVG stenosis from intimal hyperplasia.2 Stenosis of distal anastomosis of SVG or arterial grafts can be successfully dilated with balloon angioplasty as that of middle or distal portion of IMA or radial graft. Mid-SVG stenosis can also be tackled with balloon angioplasty and/or stenting, with little risk of distal embolisation. Stents and eximer laser angioplasty have been tried for the proximal anastomotic lesions with good initial results but significant rates of restenosis.
1–3 years after CABG
Patients with recurrent angina 1–3 years after CABG frequently have new stenosis in grafts and native coronary arteries that can be successfully managed with PCI. However, native lesions should be targeted whenever feasible.1
More than 3 years after CABG
At this stage, the most common cause of ischaemia is new atherosclerotic plaques in the SVG.2 These plaques are softer, more friable and larger as compared with those in native coronary arteries, and frequently have associated thrombus formation warranting the use of embolic protection.
Native coronary interventions
One year after CABG, patients develop new atherosclerotic plaques in the graft conduits, or show progression of atherosclerosis in native coronary arteries. Whenever feasible, native artery lesions are targeted for PCI because of their lower rate of restenosis, and high procedural success. Approaches to native vessel include treatment of protected left main disease, recanalisation of chronic total occlusion (CTO), or native artery via venous or arterial grafts.2
PCI of SVG
Technical strategy
The more posterior the destination of the left-sided grafts, the higher they are located on the aorta. The lowest graft usually goes to LAD, and the top one goes to distal left circumflex artery (LCX). Most left-sided grafts arise in cranial, and right coronary artery (RCA) grafts caudal from the aorta. The Judkins right coronary, left venous bypass or hockey stick catheters provide best backup support for a graft arising anteriorly (the LAD and diagonals). However, the left Amplatz and the hockey stick guides are effective for grafts arising in the inner curvature of the aorta (to the LCX). The multipurpose guide is the best for providing excellent coaxial alignment for the grafts arising from the outer curve of the aorta (usually to the RCA). If the aorta is dilated, a posteriorly located RCA graft might require Amplatz left guide for excellent backup.
Totally occluded SVG
Despite the high use of drug-eluting stent (DES), successful recanalisation of CTO of SVG is low.9 Given the poor short-term and long-term outcomes, PCI should rarely be considered in this subset except for acute occlusion in the setting of myocardial infarction (MI). Rather, recanalisation of native coronary artery is preferred.
Adjunctive pharmacotherapy
Accepted adjunctive therapy during SVG PCI includes aspirin, other antiplatelet agents, antithrombin and vasodilators. Unfractionated heparin was used in a vast majority of SVG studies. During SVG PCI, as compared with heparin, bivaluridin revealed no difference in death, MI, urgent revascularisation, or major bleeding, but there was less minor bleeding with bivaluridin in one trial.10 However, increased risk of perforation with SVGs makes the use of bivaluridin less appealing. Newer antiplatelets, such as prasugrel and ticagrelor, have not been studied in SVG PCI. The role of glycoprotein IIb/IIIa antagonists is limited given their failure to demonstrate a reduction in periprocedural MI.11–13 The large embolic burden could be one of the reasons for this.14
SVG balloon angioplasty
Balloon angioplasty is considered for mid-SVG and distal insertion site lesions. Balloons are sized 1:1 to SVG and slightly oversized for dealing with restenotic lesions or for suboptimal initial results.
Stent type selection in SVG PCI
Bare metal stent
The SAVED (Saphenous Vein de Novo) trial demonstrated higher procedural success, a trend toward a reduction in angiographic restenosis, and lower major adverse cardiac events (MACE) in the bare metal stent (BMS) group as compared with balloon angioplasty.15 Since the report, the overwhelming majority of SVG intervention has been performed with stents.
Drug-eluting stent
Even if BMS improves the initial and intermediate-term outcomes, the impact is modest due to restenosis and disease progression.1 The RRISC (Reduction of Restenosis in SVGs With Cypher Sirolimus-Eluting Stent) trial demonstrated that sirolimus-eluting stents (Cordis, Warren, New Jersey) reduced late loss, the binary restenosis rate, target lesion revascularisation (TLR), and target vessel revascularisation (TVR)16 However, the DELAYED RRISC (Death and Events at Long-term Follow-Up Analysis: Extended Duration of the Reduction of Restenosis in SVGs With Cypher Stent) study reported similar rates of TVR at 3 years.17 The paclitaxel-eluting stents (Taxus, Boston Scientific, Maple Grove, Minnesota) as compared with BMS, reported a significant reduction in MACE driven by lower TLR in SOS (Stenting of SVGs) trial.18 Few meta-analyses comparing DES with BMS in SVG PCI have reported consistent results of improved efficacy with DES without any safety hazard. 19–26 The data at present indicate that DES in SVG PCI is safe; the occurrence of death or MI is less as compared with BMS, and there is no difference in stent thrombosis. DES should always be favoured over BMS in SVGs <3.5 mm in diameter, and in patients at high risk for restenosis, such as long lesions and diabetes.1
Predilatation versus direct stenting
Direct stenting has the potential benefit of trapping debris and reducing distal embolisation which might occur from repeated balloon inflations. Leborgne et al27 demonstrated that direct stenting of SVG was associated with significant reduction in creatine kinase (CK)-MB elevation and release, and fewer non-Q-wave MI. It calls for a prospective randomised trial to determine whether direct stenting versus predilatation is effective in reducing distal embolisation.
Small stent diameter
The use of undersized DES in patients with SVG lesions is associated with a reduction in the frequency of postPCI CK-MB elevation without an increase in 1-year events, as reported by Hong et al.28 The concept of undersizing the stent to reduce distal embolisation looks promising, but such a method must be balanced by higher rates of restenosis and stent thrombosis. Therefore, a prospective, randomised study is warranted to confirm such finding.
Prophylactic stenting
In view of rapid progression of SVG disease, prophylactic stenting of intermediate lesions may be recommended as compared with medical therapy alone. In VELETI (Treatment of Moderate vein Graft Lesions With Paclitaxel Drug-Eluting Stents) trial, paclitaxel-eluting stents, as compared to medical therapy alone, significantly reduced 1-year and 3-year MACE rates in moderate (30–60%) SVG lesions, supporting a strategy of plaque sealing with DES in moderate lesions of degenerated SVGs at increased risk for disease progression and adverse clinical events.29 Further studies are required to determine if this preventive approach leads to long-term benefit.
Embolic protection
Distal embolisation is common in SVG interventions. Embolic protection devices (EPD) reduce the incidence of acute MI by 40% following SVG PCI.15 Currently available EPDs include distal balloon occlusion devices, distal filter-based devices, and proximal balloon occlusion (table 1).30
Comparison of different embolic protection devices
Distal protective devices
Distal balloon occlusion of the SVG beyond the lesion creates a significant column of blood which may prevent distal embolisation. Once the intervention is over, aspiration catheter removes the contained debris before balloon deflation restoring the antegrade blood flow. SAFER (SVG angioplasty Free of Emboli, Randomised Trial) demonstrated that PercuSurge GuardWire (Medtronic, Minneapolis, Minnesota) significantly reduced the incidence of no-reflow and 30-day MACE.31 Disadvantages of Guardwire include the need to completely occlude the target SVG during stenting and aspiration leading to ischaemia, as well as limiting visualisation, a need for a relatively long parking segment distal to the lesion and inability to protect side branches.
A distal filter system is composed of a tightly wrapped filter attached to a guidewire and sheathed within a delivery catheter for placement distal to the target lesion. It can trap embolising debris while PCI is being performed. Upon completion of stenting, a retrieval catheter is advanced over the guidewire to collapse the filter and remove it along with retained debris (figure 1). Some of the advantages of the filter include ease of use, avoidance of ischaemia because of preserved coronary flow, and good visualisation to facilitate accurate stent placement. It may be preferred in patients undergoing high-risk PCI who are at risk of haemodynamic compromise. Disadvantages include a high crossing profile, inability to completely capture the debris, possible clogging of the filter, incomplete apposition, and need for long distal parking segment. In FIRE (Filter-Wire EX Randomised Evaluation) trial, the FilterWire EX (Boston Scientific) revealed similar MACE rates at 30 days as compared with GuardWire plus system.32 A variety of filters shown to be non-inferior to distal occlusion balloon, have been applied in SVG PCI. However, there may be myonecrosis despite the use of distal EPDs (table 2).
Aetiologies for distal embolisation despite the use of distal protection devices
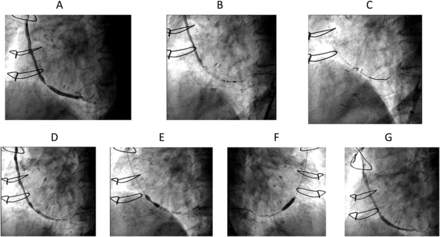
Stenting of saphenous vein graft (SVG). (A) Baseline coronary angiography revealing high-grade stenosis of SVG to obtuse marginal (OM) artery. (B) The lesion in OM is crossed with filter wire that is parked at an appropriate place. (C) Distal protection filter is opened. (D) Positioning of the stent. (E) Slowly the stent is deployed directly resembling dumbbell shape. (F) The stent is fully deployed.
Proximal protective devices
Proximal protection with Proxis system (St Jude, St Paul, Minnesota, USA) is desirable when there is insufficient parking segment beyond the lesion for distal protection. It involves placement of a hydrophilic-coated sheath into the proximal SVG. Inflation of a balloon surrounding the sheath occludes the SVG; stent implantation, followed by flow-reversal aspiration of the graft and subsequent balloon deflation, restoring flow. MACE was comparable when Proxis was compared with either FilterWire or Guardwire.33 The advantages of such EPD include the ability to institute embolic protection before crossing the lesion, to protect side branches, and handle large embolic load; also, the operator can use the guidewire of choice. The disadvantages are the inability to use the device in ostial or very proximal lesions, as 15 mm of disease-free segment proximal to the target lesion is required, and the cessation antegrade flow resulting in myocardial ischaemia.
Covered stents
This class of device is based on the concept of using specially designed stents for trapping friable atheroemboli against the arterial wall during and post-PCI. Stents covered with a mesh, most commonly polytetrafluoroethylene (PTFE) were thought to provide a useful tool for addressing distal embolisation. Unfortunately, none of the devices was able to demonstrate reduction in acute MACE, and their rate of restenosis was higher than BMS.34–38
A new stenting approach
A new plaque-trapping device, the MGuard (InspiredMD, Tel Aviv, Israel) is a novel breakthrough technology combining the clinical benefits of stent efficacy with ‘add-on’ embolic protection at the target lesion site, preprocedure and postprocedure. The MGuard design is based on a stent covered with an ultrathin, flexible mesh net fabricated by circular knitting. During stenting, the net stretches and slides over the expanding struts, creating custom-designed pores parallel to the arterial wall. In addition to embolic protection, the MGuard net diffuses strut pressure which might minimise injury to the vessel wall and reduce restenosis. Preliminary results with this stent demonstrated favourable early performance in a study that included 16 patients who underwent SVG PCI with no angiographic/procedural complications, and no adverse events up to 30 days.39 This strategy seems to be a promising approach, but it needs further validations in a large randomised trial.
Intragraft vasodilator
Slow or no-reflow is due to compromise of the integrity of the microvascular flow. Independent predictors for slow or no-reflow in SVG PCI include thrombus, lesion ulceration and degeneration of SVG. The adjunctive use of intragraft vasodilator may be promising. A variety of vasodilators have been useful in treating this condition (table 3).40 Rather than using these agents for rescue, the prophylactic administration may offer additional opportunity to reduce events. Adenosine is a vasodilator of arteries and arterioles, and inhibits platelet activation and aggregation. Prophylactic administration of adenosine does not appear to decrease the risk of slow or no-reflow, but it can reverse slow or no-reflow with multiple boluses.41 Lao L et al demonstrated that intragraft nitroprusside (median dose: 200 μg) improved angiographic flow rapidly and significantly as compared to preatreatment angiogram.42 Prophylactic intracoronary administration of verapamil tended to reduce occurrence of no-reflow compared with placebo, increased Thrombolysis In MI (TIMI) frame count and improved TIMI myocardial perfusion.43 Fischell et al44 showed promising results with nicardipine to prevent no-reflow in SVG PCI. They found that pretreatment with intragraft nicardipine, even without the use of mechanical embolic protection, resulted in low incidence of no-reflow and in-hospital MACE.
Intracoronary drug options for no reflow treatment
SVG restenosis
PCI of in-stent restenosis of SVG is safer as compared with de novo lesions because of reduction in ‘slow, no reflow’ and periprocedural MI.1 In one study, gamma radiation with 192Ir, reduced restenosis significantly as compared to placebo in in-stent restenotic lesions of SVG.45 However, DES has become the default strategy in spite of lack of data, as intracoronary brachytherapy is not available in most of the centres.
PCI of IMA grafts
PCI is feasible in left or right IMA or arterial grafts removed from the radial site. Favourable results have been reported with balloon angioplasty of IMA graft lesions. The lesions at the anastomosis occur within a few months of CABG and often respond to low-presuure balloon dilatation. Ostial, proximal and mid-segment of IMA graft may require stenting. There are paucity of data regarding stenting in IMA grafts or PCI in gastroepiploic or radial artery grafts. In PCI of IMA graft, hydrophilic steerable wire is helpful in the presence of tortuosity. Care should be taken to ensure that there is short guide length (80 cm) to reach distal sites, with extralong (145 cm) balloon catheters, or guide can be shortened and capped with a flared, short sheath, one size smaller.
Conclusion
In patients requiring bypass graft intervention, the decision making is particularly critical because of the increased risk and reduced long-term benefit. As long as SVGs are used as conduits for CABG, long-term event-free survival after this procedure will continue to be limited. Even if SVG PCI is feasible, it is risk-prone in terms of high rates of periprocedural adverse events, intermediate-term restenosis, and progression of disease outside the treatment segment. Focusing on total arterial revascularisation, or a hybrid native coronary stenting with arterial revascularisation, would minimise the need for vein graft. When SVG PCI is desirable, a proper EPD, DES undersizing and intragraft vasodilators may be useful. ‘Plaque sealing’ of moderate SVG lesions by DES, appears promising, but needs to be tested in multicenter study. Although coronary bypass graft intervention remains a formidable challenge, by focusing on simple and already proven actions, continuous physician education and technical improvements, interventionalists can make a difference in saving patients’ lives.
References
Footnotes
-
Competing interests None.
-
Provenance and peer review Not commissioned; externally peer reviewed.