Abstract
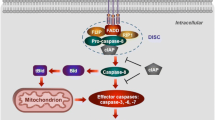
Apoptosis is an evolutionarily conserved mode of cell death that is tightly regulated and critical for multicellular organism development and cellular homeostasis. Specific biochemical and morphological changes characterise cells undergoing apoptosis, and reflect the specificity in which activated apoptotic pathways follow. The two best-characterized apoptotic pathways are the extrinsic pathway and the intrinsic pathway, which involve cell surface death receptors and the mitochondria and endoplasmic reticulum respectively. Apoptotic stimuli lead to activation of either or both of these pathways, and involve sequential activation of different cysteine proteases (caspases), and in the case of the intrinsic pathway, activation of a family of Bcl-2 proteins that critically regulate cell death. Conversely, dis-inhibition of endogenous inhibitors is often required for effective apoptotic cell death. Furthermore, an interesting recurring protein-protein interaction within this framework of apoptotic cascades involves interactions between death domain motifs that are present on many of the regulatory proteins in both apoptotic pathways. Cardiomyocyte apoptosis has been demonstrated in human heart failure and in rodents, apoptosis itself directly causes dilated cardiomyopathy. Understanding the intricacies of apoptotic death pathways and determining the relevance of these to cardiomyopathy is therefore essential if cardiomyocyte apoptosis is to be a pharmacological target for heart failure therapy.

Similar content being viewed by others
References
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116(2):205–219
Rich T, Watson CJ, Wyllie A (1999) Apoptosis: the germs of death. Nat Cell Biol 1(3):E69–E71
Virchow R (1858) Die Cellularpathologie in ihrer Begruendung auf physiologisher und pathologischer Geweblehre. Berlin
Flemming W. Uber die Bildung von Richtungsfiguren in Saeugerthiereiern beim Untergang Graaf’sscher Follikel, Archiv f Anat u Physiol. Anat Abteilung 1885:221–244
Lockshin RA, Williams CM (1964) Programmed cell death. Endocrine potentiation of the breakdown of the intersegmental muscles of silkmoths. J Insect Physiol 10:643–649
Saunders JW Jr (1966) Death in embryonic systems. Science 154(749):604–612
Kerr JF (1971) Shrinkage necrosis: a distinct mode of cellular death. J Pathol 105(1):13–20
Hengartner MO, Horvitz HR (1994) Programmed cell death in Caenorhabditis elegans. Curr Opin Genet Dev 4(4):581–586
Ashkenazi A, Dixit VM (1998) Death receptors: signaling and modulation. Science 281(5381):1305–1308
Kischkel FC, Hellbardt S, Behrmann I et al (1995) Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. Embo J 14(22):5579–5588
Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM (1998) An induced proximity model for caspase-8 activation. J Biol Chem 273(5):2926–2930
Yang X, Chang HY, Baltimore D (1998) Autoproteolytic activation of pro-caspases by oligomerization. Mol Cell 1(2):319–325
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94(4):481–490
Li H, Zhu H, Xu CJ, Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94(4):491–501
Gross A, Yin XM, Wang K et al (1999) Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem 274(2):1156–1163
Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green DR (1998) DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell 1(4):543–551
Muller M, Wilder S, Bannasch D et al (1998) p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med 188(11):2033–2045
Munsch D, Watanabe-Fukunaga R, Bourdon JC et al (2000) Human and mouse Fas (APO-1/CD95) death receptor genes each contain a p53-responsive element that is activated by p53 mutants unable to induce apoptosis. J Biol Chem 275(6):3867–3872
Pitti RM, Marsters SA, Lawrence DA et al (1998) Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 396(6712):699–703
Cheng J, Zhou T, Liu C et al (1994) Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science 263(5154):1759–1762
Cascino I, Papoff G, De Maria R, Testi R, Ruberti G (1996) Fas/Apo-1 (CD95) receptor lacking the intracytoplasmic signaling domain protects tumor cells from Fas-mediated apoptosis. J Immunol 156(1):13–17
Cascino I, Papoff G, Eramo A, Ruberti G (1996) Soluble Fas/Apo-1 splicing variants and apoptosis. Front Biosci 1:d12–d18
Tschopp J, Irmler M, Thome M (1998) Inhibition of fas death signals by FLIPs. Curr Opin Immunol 10(5):552–558
Chang L, Kamata H, Solinas G et al (2006) The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell 124(3):601–613
Holler N, Zaru R, Micheau O et al (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1(6):489–495
Siegel RM, Chan FK, Chun HJ, Lenardo MJ (2000) The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat Immunol 1(6):469–474
Badorff C, Ruetten H, Mueller S et al (2002) Fas receptor signaling inhibits glycogen synthase kinase 3 beta and induces cardiac hypertrophy following pressure overload. J Clin Invest 109(3):373–381
Foo RS, Mani K, Kitsis RN (2005) Death begets failure in the heart. J Clin Invest 115(3):565–571
Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR (2000) The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol 2(3):156–162
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305(5684):626–629
Halestrap AP (2004) Mitochondrial permeability: dual role for the ADP/ATP translocator? Nature 430(7003):1 p following 983
Woodfield K, Ruck A, Brdiczka D, Halestrap AP (1998) Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem J 336(Pt 2):287–290
Crompton M, Ellinger H, Costi A (1988) Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J 255(1):357–360
Baines CP, Kaiser RA, Purcell NH et al (2005) Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434(7033):658–662
Cory S, Adams JM (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2(9):647–656
Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR (1999) Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J Biol Chem 274(4):2225–2233
Kuwana T, Mackey MR, Perkins G et al (2002) Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111(3):331–342
Wei MC, Zong WX, Cheng EH et al (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292(5517):727–730
Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ (2002) Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2(3):183–192
Willis SN, Adams JM (2005) Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol 17(6):617–625
Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L (2003) PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci USA 100(4):1931–1936
Nakano K, Vousden KH (2001) PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 7(3):683–694
Chipuk JE, Kuwana T, Bouchier-Hayes L et al (2004) Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303(5660):1010–1014
Chipuk JE, Bouchier-Hayes L, Kuwana T, Newmeyer DD, Green DR (2005) PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science 309(5741):1732–1735
Leu JI, Dumont P, Hafey M, Murphy ME, George DL (2004) Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol 6(5):443–450
Mihara M, Erster S, Zaika A et al (2003) p53 has a direct apoptogenic role at the mitochondria. Mol Cell 11(3):577–590
Sawada M, Sun W, Hayes P, Leskov K, Boothman DA, Matsuyama S (2003) Ku70 suppresses the apoptotic translocation of Bax to mitochondria. Nat Cell Biol 5(4):320–329
Lin B, Kolluri SK, Lin F et al (2004) Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell 116(4):527–540
Konishi A, Shimizu S, Hirota J et al (2003) Involvement of histone H1.2 in apoptosis induced by DNA double-strand breaks. Cell 114(6):673–688
Li P, Nijhawan D, Budihardjo I et al (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91(4):479–489
Deveraux QL, Takahashi R, Salvesen GS, Reed JC (1997) X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388(6639):300–304
Du C, Fang M, Li Y, Li L, Wang X (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102(1):33–42
Verhagen AM, Ekert PG, Pakusch M et al (2000) Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102(1):43–53
Suzuki Y, Imai Y, Nakayama H, Takahashi K, Takio K, Takahashi R (2001) A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol Cell 8(3):613–621
Xue D, Horvitz HR (1997) Caenorhabditis elegans CED-9 protein is a bifunctional cell-death inhibitor. Nature 390(6657):305–308
Cheng EH, Kirsch DG, Clem RJ et al (1997) Conversion of Bcl-2 to a Bax-like death effector by caspases. Science 278(5345):1966–1968
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391(6662):43–50
Liu X, Zou H, Slaughter C, Wang X (1997) DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89(2):175–184
Takahashi A, Alnemri ES, Lazebnik YA et al (1996) Cleavage of lamin A by Mch2 alpha but not CPP32: multiple interleukin 1 beta-converting enzyme-related proteases with distinct substrate recognition properties are active in apoptosis. Proc Natl Acad Sci USA 93(16):8395–8400
Orth K, Chinnaiyan AM, Garg M, Froelich CJ, Dixit VM (1996) The CED-3/ICE-like protease Mch2 is activated during apoptosis and cleaves the death substrate lamin A. J Biol Chem 271(28):16443–16446
Wen LP, Fahrni JA, Troie S, Guan JL, Orth K, Rosen GD (1997) Cleavage of focal adhesion kinase by caspases during apoptosis. J Biol Chem 272(41):26056–26061
Kothakota S, Azuma T, Reinhard C et al (1997) Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science 278(5336):294–298
Shi Y (2004) Caspase activation: revisiting the induced proximity model. Cell 117(7):855–888
Pop C, Timmer J, Sperandio S, Salvesen GS (2006) The apoptosome activates caspase-9 by dimerization. Mol Cell 22(2):269–275
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281(5381):1312–1316
Lakhani SA, Masud A, Kuida K et al (2006) Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311(5762):847–851
Morita Y, Maravei DV, Bergeron L et al (2001) Caspase-2 deficiency prevents programmed germ cell death resulting from cytokine insufficiency but not meiotic defects caused by loss of ataxia telangiectasia-mutated (Atm) gene function. Cell Death Differ 8(6):614–620
Robertson JD, Enoksson M, Suomela M, Zhivotovsky B, Orrenius S (2002) Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J Biol Chem 277(33):29803–29809
Lassus P, Opitz-Araya X, Lazebnik Y (2002) Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science 297(5585):1352–1354
Tinel A, Tschopp J (2004) The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science 304(5672):843–846
Kaufman RJ (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13(10):1211–1233
Iwawaki T, Hosoda A, Okuda T et al (2001) Translational control by the ER transmembrane kinase/ribonuclease IRE1 under ER stress. Nat Cell Biol 3(2):158–164
Urano F, Wang X, Bertolotti A et al (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287(5453):664–666
Ma K, Vattem KM, Wek RC (2002) Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. J Biol Chem 277(21):18728–18735
Nishitoh H, Matsuzawa A, Tobiume K et al (2002) ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 16(11):1345–13555
Yamaguchi O, Higuchi Y, Hirotani S et al (2003) Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc Natl Acad Sci USA 100(26):15883–15888
Hetz C, Bernasconi P, Fisher J et al (2006) Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science 312(5773):572–576
Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1(1):11–21
Oakes SA, Scorrano L, Opferman JT et al (2005) Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci USA 102(1):105–110
Szalai G, Krishnamurthy R, Hajnoczky G (1999) Apoptosis driven by IP(3)-linked mitochondrial calcium signals. Embo J 18(22):6349–6361
Nakagawa T, Zhu H, Morishima N et al (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403(6765):98–103
Yoneda T, Imaizumi K, Oono K et al (2001) Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem 276(17):13935–13940
Nakagawa T, Yuan J (2000) Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol 150(4):887–894
Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y (2002) An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem 277(37):34287–34294
Saleh M, Vaillancourt JP, Graham RK et al (2004) Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature 429(6987):75–79
Fliss H, Gattinger D (1996) Apoptosis in ischemic and reperfused rat myocardium. Circ Res 79(5):949–956
Lee P, Sata M, Lefer DJ, Factor SM, Walsh K, Kitsis RN (2003) Fas pathway is a critical mediator of cardiac myocyte death and MI during ischemia-reperfusion in vivo. Am J Physiol Heart Circ Physiol 284(2):H456–H463
Jeremias I, Kupatt C, Martin-Villalba A et al (2000) Involvement of CD95/Apo1/Fas in cell death after myocardial ischemia. Circulation 102(8):915–920
Guerra S, Leri A, Wang X et al (1999) Myocyte death in the failing human heart is gender dependent. Circ Res 85(9):856–866
Olivetti G, Abbi R, Quaini F et al (1997) Apoptosis in the failing human heart. N Engl J Med 336(16):1131–1141
Saraste A, Pulkki K, Kallajoki M et al (1999) Cardiomyocyte apoptosis and progression of heart failure to transplantation. Eur J Clin Invest 29(5):380–386
Wencker D, Chandra M, Nguyen K et al (2003) A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest 111(10):1497–1504
D’Angelo DD, Sakata Y, Lorenz JN et al (1997) Transgenic Galphaq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci USA 94(15):8121–8126
Adams JW, Sakata Y, Davis MG et al (1998) Enhanced Galphaq signaling: a common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci USA 95(17):10140–10145
Yussman MG, Toyokawa T, Odley A et al (2002) Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nat Med 8(7):725–730
Hayakawa Y, Chandra M, Miao W et al (2003) Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of Galpha(q) transgenic mice. Circulation 108(24):3036–3041
Chatterjee S, Stewart AS, Bish LT et al (2002) Viral gene transfer of the antiapoptotic factor Bcl-2 protects against chronic postischemic heart failure. Circulation 106(12 Suppl 1):I212–I217
Chatterjee S, Bish LT, Jayasankar V et al (2003) Blocking the development of postischemic cardiomyopathy with viral gene transfer of the apoptosis repressor with caspase recruitment domain. J Thorac Cardiovasc Surg 125(6):1461–1469
Hirota H, Chen J, Betz UA et al (1999) Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell 97(2):189–198
Donath S, Li P, Willenbockel C et al (2006) Apoptosis repressor with caspase recruitment domain is required for cardioprotection in response to biomechanical and ischemic stress. Circulation 113(9):1203–1212
Ide T, Tsutsui H, Kinugawa S et al (2000) Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ Res 86(2):152–157
Kajstura J, Cigola E, Malhotra A et al (1997) Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol 29(3):859–870
Shizukuda Y, Buttrick PM, Geenen DL, Borczuk AC, Kitsis RN, Sonnenblick EH (1998) beta-adrenergic stimulation causes cardiocyte apoptosis: influence of tachycardia and hypertrophy. Am J Physiol 275(3 Pt 2):H961–H968
Communal C, Singh K, Pimentel DR, Colucci WS (1998) Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation 98(13):1329–1334
Mann DL (1999) Inflammatory mediators in heart failure: homogeneity through heterogeneity. Lancet 353(9167):1812–1813
Cheng W, Li B, Kajstura J et al (1995) Stretch-induced programmed myocyte cell death. J Clin Invest 96(5):2247–2259
Oh H, Taffet GE, Youker KA et al (2001) Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc Natl Acad Sci USA 98(18):10308–10313
Oh H, Wang SC, Prahash A et al (2003) Telomere attrition and Chk2 activation in human heart failure. Proc Natl Acad Sci USA 100(9):5378–5383
Okada K, Minamino T, Tsukamoto Y et al (2004) Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 110(6):705–712
Communal C, Sumandea M, de Tombe P, Narula J, Solaro RJ, Hajjar RJ (2002) Functional consequences of caspase activation in cardiac myocytes. Proc Natl Acad Sci USA 99(9):6252–6256
Narula J, Pandey P, Arbustini E et al (1999) Apoptosis in heart failure: release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci USA 96(14):8144–8149
Haider N, Narula N, Narula J (2002) Apoptosis in heart failure represents programmed cell survival, not death, of cardiomyocytes and likelihood of reverse remodeling. J Card Fail 8(6 Suppl):S512–S517
Narula J, Haider N, Arbustini E, Chandrashekhar Y (2006) Mechanisms of disease: apoptosis in heart failure–seeing hope in death. Nat Clin Pract Cardiovasc Med 3(12):681–688
Acknowledgement
R.S-Y.Foo is supported by a Wellcome Trust Advanced Fellowship. MM is supported by a project grant from the British Heart Foundation to RSYF and Professor Martin Bennett (Cambridge).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Movassagh, M., Foo, R.SY. Simplified apoptotic cascades. Heart Fail Rev 13, 111–119 (2008). https://doi.org/10.1007/s10741-007-9070-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-007-9070-x