Abstract
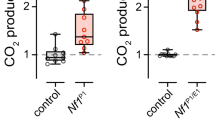
Neurofibromatosis type 1 (NF1) is a dominant genetic disorder characterized by multiple benign and malignant nervous system tumors, and by learning defects in 45% of children with NF1 mutations. Studies of neurofibromin, the protein encoded by NF1, have focused on its functions in tumorigenesis and regulation of Ras activity; however, Drosophila NF1 regulates both Ras and cyclic AMP (cAMP) pathways. Expression of a human NF1 transgene rescued cAMP-related phenotypes in NF1 mutant flies (small body size and G protein–stimulated adenylyl cyclase (AC) activity defects), and neuropeptide– and G protein–stimulated AC activity were lower in Nf1−/− as compared to Nf1+/− mouse brains, demonstrating that neurofibromin regulates AC activity in both mammals and flies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cichowski, K. et al. Science 286, 2172–2176 (1999).
Vogel, K. S. et al. Science 286, 2176–2179 (1999).
Silva, A. J. et al. Nat. Genet. 15, 281–284 (1997).
Costa, R. M. et al. Nat Genet. 27, 399–405 (2001).
Guo, H. F., Tong, J., Hannan, F., Luo, L. & Zhong, Y. Nature 403, 895–898 (2000).
Fahsold, R. et al. Am. J. Hum. Genet. 66, 790–818 (2000).
Kim, H. A., Ling, B. & Ratner, N. Mol. Cell. Biol. 17, 862–872 (1997).
Hsueh, Y. P., Roberts, A. M., Volta, M., Sheng, M. & Roberts, R. G. J. Neurosci . 21, 3764–3770 (2001).
Guo, H. F., The, I., Hannan, F., Bernards, A. & Zhong, Y. Science 276, 795–798 (1997).
The, I. et al. Science 276, 791–794 (1997).
Williams, J. A., Su, H. S., Bernards, A., Field, J. & Sehgal, A. Science 293, 2251–2256 (2001).
Brannan, C. et al. Genes Dev. 8, 1019–1029 (1994).
Levin, L. R. et al. Cell 68, 479–489 (1992).
Defer, N., Best-Belpomme, M. & Hanoune, J. Am. J. Physiol. Renal Physiol. 279, F400–F416 (2000).
Acknowledgements
We thank I. Hakker, J. An and J. Coblentz for technical help. This work was supported by grants to Y.Z. from the US National Institutes of Health (NS34779), US Army (DAMD17-99-1-9500), Neurofibromatosis Foundation, Massachusetts Bay area and Neurofibromatosis Foundation, Illinois.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Developmental AC activity profiles. AC activity was assayed using membrane fractions extracted from mouse frontal lobes at each developmental stage, including embryonic day 12.5 (E12.5), postnatal day 1 (P1) and adult (3 month). All data were obtained from wild-type mice. Extracts from each individual were divided into three; number of assays for each data point, n = 3. Data are expressed as mean ± s.e.m.. Stimulated AC activities were significantly higher in all situations (p < 0.01; two-tailed Student's t-test). (GIF 26 kb)
Supplementary Figure 2
Similar expression levels of the stimulatory G protein a-subunit in Nf1 knockout versus wild-type mice. A representative example of immunoblots of Gsa with antisera from Calbiochem (1:1,000) after SDS-PAGE electrophoresis is shown. No obvious disruption was seen. Frontal brain tissues from Nf1+/+, Nf1+/- and Nf1-/- littermates were homogenized for western blot analysis. Molecular-weight markers are indicated. Three independent analyses were performed. (JPG 5 kb)
Rights and permissions
About this article
Cite this article
Tong, J., Hannan, F., Zhu, Y. et al. Neurofibromin regulates G protein–stimulated adenylyl cyclase activity. Nat Neurosci 5, 95–96 (2002). https://doi.org/10.1038/nn792
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn792
This article is cited by
-
Periampullary tumors in a patient with pancreatic divisum and neurofibromatosis type 1: a case report
Hereditary Cancer in Clinical Practice (2023)
-
The therapeutic potential of neurofibromin signaling pathways and binding partners
Communications Biology (2023)
-
Using antisense oligonucleotides for the physiological modulation of the alternative splicing of NF1 exon 23a during PC12 neuronal differentiation
Scientific Reports (2021)
-
Neurofibromin 1 in mushroom body neurons mediates circadian wake drive through activating cAMP–PKA signaling
Nature Communications (2021)
-
Traffic lights for retinoids in oncology: molecular markers of retinoid resistance and sensitivity and their use in the management of cancer differentiation therapy
BMC Cancer (2018)