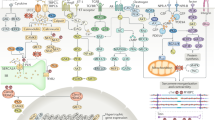
Key Points
-
Interactions between the heart and kidney form the basis of cardiorenal syndrome, which is a heterogeneous and complex clinical entity associated with substantial morbidity and mortality
-
Precise clinical characterization and classification of cardiorenal syndrome has not yet been performed
-
The factors that mediate connections between the heart and the kidney and their complex interactions must be clarified in vitro and in experimental models before clinical applications are sought
-
Iron metabolism and erythrocyte turnover are likely to be central to the pathophysiology of cardiorenal syndrome
Abstract
Combined cardiac and renal dysfunction has gained considerable attention. Hypotheses about its pathogenesis have been formulated, albeit based on a relatively small body of experimental studies, and a clinical classification system has been proposed. Cardiorenal syndrome, as presently defined, comprises a heterogeneous group of acute and chronic clinical conditions, in which the failure of one organ (heart or kidney) initiates or aggravates failure of the other. This conceptual framework, however, has two major drawbacks: the first is that, despite worldwide interest, universally accepted definitions of cardiorenal syndrome are lacking and characterization of heart and kidney failure is not uniform. This lack of consistency hampers experimental studies on mechanisms of the disease. The second is that, although progress has been made in developing hypotheses for the pathogenesis of cardiorenal syndrome, these initiatives are at an impasse. No hierarchy has been identified in the myriad of haemodynamic and non-haemodynamic factors mediating cardiorenal syndrome. This Review discusses current understanding of cardiorenal syndrome and provides a roadmap for further studies in this field. Ultimately, discussion of the definition and characterization issues and of the lack of organization among pathogenetic factors is hoped to contribute to further advancement of this complex field.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bongartz, L. G., Cramer, M. J., Doevendans, P. A., Joles, J. A. & Braam, B. The severe cardiorenal syndrome: 'Guyton revisited'. Eur. Heart J. 26, 11–17 (2005).
El Nahas, M. Cardio-Kidney-Damage: a unifying concept. Kidney Int. 78, 14–18 (2010).
Ronco, C., Haapio, M., House, A. A., Anavekar, N. & Bellomo, R. Cardiorenal syndrome. J. Am. Coll. Cardiol. 52, 1527–1539 (2008).
Damman, K. et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J. Card. Fail. 13, 599–608 (2007).
Earle, D. P. Jr, Farber, S. J., Alexander, J. D. & Eichna, L. W. Effect of treatment on renal functions and electrolyte excretion in congestive heart failure. J. Clin. Invest. 28, 778 (1949).
Futcher, P. H. & Schroeder, H. A. Studies on congestive heart failure. II. Impaired renal excretion of sodium chloride. Am. J. Med. Sci. 204, 52 (1942).
Seymour, W. B., Pritchard, W. H., Longley, L. P. & Hayman, J. M. Cardiac output, blood and interstitial fluid volumes, total circulating serum protein, and kidney function during cardiac failure and after improvement. J. Clin. Invest. 21, 229–240 (1942).
Winton, F. R. The influence of venous pressure on the isolated mammalian kidney. J. Physiol. 72, 49–61 (1931).
Guyton, A. C. The surprising kidney-fluid mechanism for pressure control—its infinite gain! Hypertension 16, 725–730 (1990).
Schwarz, U. et al. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol. Dial. Transplant. 15, 218–223 (2000).
Tornig, J. et al. Hypertrophy of intramyocardial arteriolar smooth muscle cells in experimental renal failure. J. Am. Soc. Nephrol. 10, 77–83 (1999).
Schwarz, U., Amann, K. & Ritz, E. Why are coronary plaques more malignant in the uraemic patient? Nephrol. Dial. Transplant. 14, 224–225 (1999).
Amann, K., Breitbach, M., Ritz, E. & Mall, G. Myocyte/capillary mismatch in the heart of uremic patients. J. Am. Soc. Nephrol. 9, 1018–1022 (1998).
Bongartz, L. G. et al. Transient nitric oxide reduction induces permanent cardiac systolic dysfunction and worsens kidney damage in rats with chronic kidney disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R815–R823 (2010).
Scadding, J. G. Diagnosis: the clinician and the computer. Lancet 2, 877–882 (1967).
Bock, J. S. & Gottlieb, S. S. Cardiorenal syndrome: new perspectives. Circulation 121, 2592–2600 (2010).
Bongartz, L. G. et al. Target organ crosstalk in the cardiorenal syndrome: animal models. Am. J. Physiol. Renal Physiol. 303, F1253–F1263 (2012).
Wright, H. J. & MacAdam, D. B. Clinical thinking and practice: diagnosis and decision in patient care (Churchill Livingstone, 1979).
Damman, K. et al. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J. Am. Coll. Cardiol. 53, 582–588 (2009).
Mullens, W. et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 53, 589–596 (2009).
Uthoff, H. et al. Central venous pressure and impaired renal function in patients with acute heart failure. Eur. J. Heart Fail. 13, 432–439 (2011).
Hatamizadeh, P. et al. Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat. Rev. Nephrol. 9, 99–111 (2013).
Testani, J. M., Kimmel, S. E., Dries, D. L. & Coca, S. G. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ. Heart Fail. 4, 685–691 (2011).
Bellomo, R. et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit. Care 8, R204–R212 (2004).
Ronco, C. et al. Improving outcomes from acute kidney injury (AKI): report on an initiative. Int. J. Artif. Organs 30, 373–376 (2007).
McCullough P. A. et al. Prevention of cardio-renal syndromes: workgroup statements from the 7th ADQI consensus conference. Nephrol. Dial. Transplant. 25, 1777–1784 (2010).
Bagshaw, S. M. et al. Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI consensus conference. Nephrol. Dial. Transplant. 25, 1406–1416 (2010).
Gottschalk, C. W. & Mylle, M. Micropuncture study of pressures in proximal tubules and peritubular capillaries of the rat kidney and their relation to ureteral and renal venous pressures. Am. J. Physiol. 185, 430–439 (1956).
Deen, W. M., Robertson, C. R. & Brenner, B. M. A model of glomerular ultrafiltration in the rat. Am. J. Physiol. 223, 1178–1183 (1972).
Cannon, P. J. The kidney in heart failure. N. Engl. J. Med. 296, 26–32 (1977).
Stanton, R. C. & Brenner, B. M. Role of the kidney in congestive heart failure. Acta Med. Scand. Suppl. 707, 21–25 (1986).
Ito, S. Cardiorenal syndrome: an evolutionary point of view. Hypertension 60, 589–595 (2012).
Silverberg, D. S. et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J. Am. Coll. Cardiol. 35, 1737–1744 (2000).
Cogan, M. G. Angiotensin II: a powerful controller of sodium transport in the early proximal tubule. Hypertension 15, 451–458 (1990).
Braam, B., Cupples, W. A., Joles, J. A. & Gaillard, C. Systemic arterial and venous determinants of renal hemodynamics in congestive heart failure. Heart Fail. Rev. 17, 161–175 (2012).
Kirchheim, H., Ehmke, H. & Persson, P. Sympathetic modulation of renal hemodynamics, renin release and sodium excretion. Klin. Wochenschr. 67, 858–864 (1989).
Kishimoto, T., Maekawa, M., Abe, Y. & Yamamoto, K. Intrarenal distribution of blood flow and renin release during renal venous pressure elevation. Kidney Int. 4, 259–266 (1973).
Kopp, U. C., Olson, L. A. & DiBona, G. F. Renorenal reflex responses to mechano- and chemoreceptor stimulation in the dog and rat. Am. J. Physiol. 246, F67–F77 (1984).
Skott, O. & Briggs, J. P. Direct demonstration of macula densa-mediated renin secretion. Science 237, 1618–1620 (1987).
Tornig, J. et al. Arteriolar wall thickening, capillary rarefaction and interstitial fibrosis in the heart of rats with renal failure: the effects of ramipril, nifedipine and moxonidine. J. Am. Soc. Nephrol. 7, 667–675 (1996).
Crawford, D. C., Chobanian, A. V. & Brecher, P. Angiotensin II induces fibronectin expression associated with cardiac fibrosis in the rat. Circ. Res. 74, 727–739 (1994).
Groeschel, M. & Braam, B. Connecting chronic and recurrent stress to vascular dysfunction: no relaxed role for the renin-angiotensin system. Am. J. Physiol. Renal Physiol. 300, F1–F10 (2011).
Ligtenberg, G. et al. Reduction of sympathetic hyperactivity by enalapril in patients with chronic renal failure. N. Engl. J. Med. 340, 1321–1328 (1999).
Chen, H. H., Redfield, M. M., Nordstrom, L. J., Cataliotti, A. & Burnett, J. C. Jr. Angiotensin II AT1 receptor antagonism prevents detrimental renal actions of acute diuretic therapy in human heart failure. Am. J. Physiol. Renal Physiol. 284, F1115–F1119 (2003).
Schlaich, M. P. et al. Sympathetic activation in chronic renal failure. J. Am. Soc. Nephrol. 20, 933–939 (2009).
Joles, J. A. & Koomans, H. A. Causes and consequences of increased sympathetic activity in renal disease. Hypertension 43, 699–706 (2004).
Koomans, H. A., Blankestijn, P. J. & Joles, J. A. Sympathetic hyperactivity in chronic renal failure: a wake-up call. J. Am. Soc. Nephrol. 15, 524–537 (2004).
U.S. National Library of Medicine. ClinicalTrials.gov [online], (2013).
Modlinger, P. S., Wilcox, C. S. & Aslam, S. Nitric oxide, oxidative stress, and progression of chronic renal failure. Semin. Nephrol. 24, 354–365 (2004).
Braam, B. Renal endothelial and macula densa NOS: integrated response to changes in extracellular fluid volume. Am. J. Physiol. 276, R1551–R1561 (1999).
Turkstra, E., Braam, B. & Koomans, H. A. Nitric oxide release as an essential mitigating step in tubuloglomerular feedback: observations during intrarenal nitric oxide clamp. J. Am. Soc. Nephrol. 9, 1596–1603 (1998).
Turkstra, E., Braam, B. & Koomans, H. A. Impaired renal blood flow autoregulation in two-kidney, one-clip hypertensive rats is caused by enhanced activity of nitric oxide. J. Am. Soc. Nephrol. 11, 847–855 (2000).
Welch, W. J., Mendonca, M., Aslam, S. & Wilcox, C. S. Roles of oxidative stress and AT1 receptors in renal hemodynamics and oxygenation in the postclipped 2K, 1C kidney. Hypertension 41, 692–696 (2003).
Wilcox, C. S. Redox regulation of the afferent arteriole and tubuloglomerular feedback. Acta Physiol. Scand. 179, 217–223 (2003).
Rosenbaugh, E. G., Savalia, K. K., Manickam, D. S. & Zimmerman, M. C. Antioxidant-based therapies for angiotensin II-associated cardiovascular diseases. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R917–R928 (2013).
Schmidt, R. J. & Baylis, C. Total nitric oxide production is low in patients with chronic renal disease. Kidney Int. 58, 1261–1266 (2000).
Sharma, R. & Davidoff, M. N. Oxidative stress and endothelial dysfunction in heart failure. Congest. Heart Fail. 8, 165–172 (2002).
Bongartz, L. G. et al. Subtotal nephrectomy plus coronary ligation leads to more pronounced damage in both organs than either nephrectomy or coronary ligation. Am. J. Physiol. Heart Circ. Physiol. 302, H845–H854 (2012).
Bongartz, L. G. et al. The nitric oxide donor molsidomine rescues cardiac function in rats with chronic kidney disease and cardiac dysfunction. Am. J. Physiol. Heart Circ. Physiol. 299, H2037–H2045 (2010).
Ramos, L. F. et al. Effects of combination tocopherols and alpha lipoic acid therapy on oxidative stress and inflammatory biomarkers in chronic kidney disease. J. Ren. Nutr. 21, 211–218 (2011).
Chae, C. U., Albert, C. M., Moorthy, M. V., Lee, I. M. & Buring, J. E. Vitamin E supplementation and the risk of heart failure in women. Circ. Heart Fail. 5, 176–182 (2012).
Robinson, I., de Serna, D. G., Gutierrez, A. & Schade, D. S. Vitamin E in humans: an explanation of clinical trial failure. Endocr. Pract. 12, 576–582 (2006).
Jun, M. et al. Antioxidants for chronic kidney disease. Cochrane Database of Systematic Reviews, Issue 10, Art. No.:CD008176. http://dx.doi.org/10.1002/14651858.CD008176.pub2.
Boengler, K., Hilfiker-Kleiner, D., Drexler, H., Heusch, G. & Schulz, R. The myocardial JAK/STAT pathway: from protection to failure. Pharmacol. Ther. 120, 172–185 (2008).
Kisseleva, T., Bhattacharya, S., Braunstein, J. & Schindler, C. W. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 285, 1–24 (2002).
Murray, P. J. The JAK-STAT signaling pathway: input and output integration. J. Immunol. 178, 2623–2629 (2007).
Heinrich, P. C. et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1–20 (2003).
Bluyssen, H. A. et al. IFN gamma-dependent SOCS3 expression inhibits IL-6-induced STAT3 phosphorylation and differentially affects IL-6 mediated transcriptional responses in endothelial cells. Am. J. Physiol. Cell. Physiol. 299, C354–C362 (2010).
Wegrzyn, J. et al. Function of mitochondrial Stat3 in cellular respiration. Science 323, 793–797 (2009).
Krebs, D. L. & Hilton, D. J. SOCS proteins: negative regulators of cytokine signaling. Stem Cells 19, 378–387 (2001).
Alexander, W. S. et al. Suppressors of cytokine signaling (SOCS): negative regulators of signal transduction. J. Leukoc. Biol. 66, 588–592 (1999).
Starr, R. & Hilton, D. J. Negative regulation of the JAK/STAT pathway. Bioessays 21, 47–52 (1999).
Chen, W., Daines, M. O. & Khurana Hershey, G. K. Turning off signal transducer and activator of transcription (STAT): the negative regulation of STAT signaling. J. Allergy Clin. Immunol. 114, 476–489 (2004).
Croker, B. A. et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat. Immunol. 4, 540–545 (2003).
Gelinas, L., Falkenham, A., Oxner, A., Sopel, M. & Legare, J. F. Highly purified human peripheral blood monocytes produce IL-6 but not TNFα in response to angiotensin II. J. Renin Angiotensin Aldosterone Syst. 12, 295–303 (2011).
Sanceau, J., Wijdenes, J., Revel, M. & Wietzerbin, J. IL-6 and IL-6 receptor modulation by IFN-gamma and tumor necrosis factor-alpha in human monocytic cell line (THP-1). Priming effect of IFN-gamma. J. Immunol. 147, 2630–2637 (1991).
Negoro, S. et al. Activation of JAK/STAT pathway transduces cytoprotective signal in rat acute myocardial infarction. Cardiovasc. Res. 47, 797–805 (2000).
Hirota, H. et al. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell 97, 189–198 (1999).
Hattori, R. et al. Role of STAT3 in ischemic preconditioning. J. Mol. Cell. Cardiol. 33, 1929–1936 (2001).
Stephanou, A. et al. Ischemia-induced STAT-1 expression and activation play a critical role in cardiomyocyte apoptosis. J. Biol. Chem. 275, 10002–10008 (2000).
Go, A. S. et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the anemia in chronic heart failure: outcomes and resource utilization (ANCHOR) study. Circulation 113, 2713–2723 (2006).
Silverberg, D., Wexler, D., Blum, M., Wollman, Y. & Iaina, A. The cardio-renal anaemia syndrome: does it exist? Nephrol. Dial. Transplant. 18 (Suppl. 8), viii7–viii12 (2003).
Silverberg, D. S., Wexler, D., Iaina, A. & Schwartz, D. The interaction between heart failure and other heart diseases, renal failure, and anemia. Semin. Nephrol. 26, 296–306 (2006).
Jie, K. E. et al. Erythropoietin and the cardiorenal syndrome: cellular mechanisms on the cardiorenal connectors. Am. J. Physiol. Renal Physiol. 291, F932–F944 (2006).
van der Putten, K., Braam, B., Jie, K. E. & Gaillard, C. A. Mechanisms of disease: erythropoietin resistance in patients with both heart and kidney failure. Nat. Clin. Pract. Nephrol. 4, 47–57 (2008).
Drueke, T. B. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N. Engl. J. Med. 355, 2071–2084 (2006).
Singh, A. K. et al. Correction of anemia with epoetin alfa in chronic kidney disease. N. Engl. J. Med. 355, 2085–2098 (2006).
Pfeffer, M. A. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N. Engl. J. Med. 361, 2019–2032 (2009).
Swedberg, K. et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N. Engl. J. Med. 368, 1210–1219 (2013).
Kobori, H. et al. Young scholars award lecture: intratubular angiotensinogen in hypertension and kidney diseases. Am. J. Hypertens. 19, 541–550 (2006).
Kainer, R. A functional model of the rat kidney. J. Math. Biol. 7, 57–94 (1979).
Jensen, P. K., Christensen, O. & Steven, K. A mathematical model of fluid transport in the kidney. Acta Physiol. Scand. 112, 373–385 (1981).
van der Lubbe, N. et al. Angiotensin II induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter independent of aldosterone. Kidney Int. 79, 66–76 (2011).
Gaillard, C. A. & Schiffelers, R. M. Red blood cell: barometer of cardiovascular health? Cardiovasc. Res. 98, 3–4 (2013).
van der Putten, K. et al. Erythropoietin treatment in patients with combined heart and renal failure: objectives and design of the EPOCARES study. J. Nephrol. 23, 363–368 (2010).
van der Putten, K. et al. Hepcidin-25 is a marker of the response rather than resistance to exogenous erythropoietin in chronic kidney disease/chronic heart failure patients. Eur. J. Heart Fail. 12, 943–950 (2010).
Emans, M. E. et al. Determinants of red cell distribution width (RDW) in cardiorenal patients: RDW is not related to erythropoietin resistance. J. Card. Fail. 17, 626–633 (2011).
Emans, M. E. et al. Red cell distribution width is associated with physical inactivity and heart failure, independent of established risk factors, inflammation or iron metabolism; the EPIC-Norfolk study. Int. J. Cardiol. 168, 3550–3555 (2013).
Klip, I. T. et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am. Heart J. 165, 575–582 (2013).
Anker, S. D. et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl. J. Med. 361, 2436–2448 (2009).
Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2, 279–335 (2012).
Gut, N. et al. Erythropoietin combined with ACE inhibitor prevents heart remodeling in 5/6 nephrectomized rats independently of blood pressure and kidney function. Am. J. Nephrol. 38, 124–135 (2013).
Massie, B. M. et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N. Engl. J. Med. 363, 1419–1428 (2010).
Shlipak, M. G. & Massie, B. M. The clinical challenge of cardiorenal syndrome. Circulation 110, 1514–1517 (2004).
Heywood, J. T. The cardiorenal syndrome: lessons from the ADHERE database and treatment options. Heart Fail. Rev. 9, 195–201 (2004).
Acknowledgements
B. Braam is a Heart and Stroke Foundation of Canada New Investigator and a Mazankowski Alberta Heart Institute member.
Author information
Authors and Affiliations
Contributions
B. Braam and A. H. Danishwar researched the data for the article and wrote the manuscript. B. Braam, J. A. Joles and C. A. Gaillard contributed substantially to discussions of the article's content. All four authors contributed to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Braam, B., Joles, J., Danishwar, A. et al. Cardiorenal syndrome—current understanding and future perspectives. Nat Rev Nephrol 10, 48–55 (2014). https://doi.org/10.1038/nrneph.2013.250
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2013.250
This article is cited by
-
The gut microbiome tango in the progression of chronic kidney disease and potential therapeutic strategies
Journal of Translational Medicine (2023)
-
The cardio-renal-metabolic connection: a review of the evidence
Cardiovascular Diabetology (2023)
-
Type 2 Myocardial Infarction and Long-Term Mortality Risk Factors: A Retrospective Cohort Study
Advances in Therapy (2023)
-
Cardiovascular Alterations and Structural Changes in the Setting of Chronic Kidney Disease: a Review of Cardiorenal Syndrome Type 4
SN Comprehensive Clinical Medicine (2022)
-
Preexisting heart failure with reduced ejection fraction attenuates renal fibrosis after ischemia reperfusion via sympathetic activation
Scientific Reports (2021)