Article Text
Abstract
Objective Neoatherosclerosis or atherosclerosis progression is one of the mechanisms of long-term stent failure. Yellow plaque detected by angioscopy has been associated with advanced atherosclerosis and the future risk of a coronary event. We compared the yellow colour of the stented segment between zotarolimus-eluting stents (ZES) and everolimus-eluting stents (EES) at 1 year after implantation.
Design Cross-sectional study.
Patients Consecutive patients underwent angioscopic examination 1 year after the implantation of ZES (n=45) or EES (n=45) at a de novo native coronary lesion.
Main outcome measures The maximum yellow colour grade (grade 0–3) of the stented segment, maximum and minimum neointima coverage grade (grade 0–2) and the presence of thrombus were examined. The neointima heterogeneity index was calculated as maximum − minimum coverage grade.
Results Maximum yellow colour grade was higher in EES than in ZES (1.3±0.9 vs 0.4±0.8, p<0.001) and maximum (2.0±0.2 vs 1.2±0.5, p<0.001) and minimum (1.5±0.6 vs 0.7±0.5, p<0.001) coverage grade was higher in ZES than in EES. The neointima heterogeneity index was not different between ZES and EES (0.4±0.5 vs 0.5±0.6, p=0.42). The incidence of thrombus was very low and was not different between ZES and EES (2% vs 4%, p=0.55).
Conclusions Although both ZES and EES had good healing with homogeneous neointima coverage and a low incidence of thrombus, EES had more advanced atherosclerosis as shown by the presence of higher grade yellow plaque than ZES at 1 year after implantation.
Statistics from Altmetric.com
Compared with bare metal stents (BMS), drug-eluting stents (DES) have reduced early target lesion revascularisation (TLR) through an inhibitory effect on neointima hyperplasia but have increased the risk of stent thrombosis and TLR after 1 year (ie, late stent failure).1 Although the incidence of stent thrombosis and TLR appears to be reduced with the newer DES than with the first-generation DES,2–8 the mechanisms of late stent failure are not well understood.
Angioscopy, as a tool of macroscopic pathology in living patients, has revealed the process of vessel response against BMS or DES implantation.9–21 It can evaluate the healing response after stent implantation by the grade of neointima coverage and incidence of thrombus. Furthermore, it can evaluate the extent of atherosclerosis by the yellow colour intensity of the lesion. Yellow plaques, especially those of high yellow colour grade, are regarded as vulnerable and have been associated with future coronary events.22–25 The sirolimus-eluting stent (SES) is known to remain thrombogenic for years and, thus, delayed healing is an important mechanism for late stent failure in SES. On the other hand, neoatherosclerosis has been reported as a cause of late stent failure in both DES and BMS.26 In the present study we compared the extent of atherosclerosis, as shown by the yellow colour grade of the stented segment, between zotarolimus-eluting stents (ZES) and everolimus-eluting stents (EES) at 1 year after implantation.
Methods
Study design
From May 2010 to July 2012 we included consecutive patients who underwent catheterisation and angioscopic examination at 1 year after the implantation of ZES (Endeavor stent; Medtronic, Minneapolis, USA) (n=45) or EES (Xience V; Abbott Vascular, Santa Clara, USA) (n=45) at de novo lesions of native coronary arteries. Patients with in-stent restenosis (>75%) at follow-up were excluded. Catheterisation was performed by the femoral, brachial or radial artery approach using a 6 Fr sheath and catheters. A coronary angiogram was recorded by the Innova Cardiovascular imaging system (GE Healthcare Japan, Tokyo, Japan) and quantitative coronary angiographic analysis was performed. All patients were taking aspirin 100 mg/day and ticlopidine 500 mg/day or clopidogrel 75 mg/day (dual antiplatelet therapy) throughout the study period. GPIIb/IIIa inhibitors were not used because they are not approved in Japan for clinical use. Hypertensive patients were defined as patients with blood pressure >140/90 mm Hg or those already taking antihypertensive drugs. Diabetic patients were defined as patients with fasting blood glucose >126 mg/dL or those already taking oral drugs for diabetes mellitus or receiving insulin therapy. Acute coronary syndrome includes acute myocardial infarction with or without ST elevation defined by the Joint European Society of Cardiology/American College of Cardiology Committee and unstable angina defined according to the Braunwald classification.
Angioscopic examination and evaluation
The angioscope RX-3310A and MV-5010A (Machida, Tokyo, Japan) and optic fibre DAG-2218 LN (Machida, Tokyo, Japan) were used. Angioscopic observation of stented segments was done while blood was cleared from view by the injection of 3% dextran-40 as previously reported.9–12 ,27 Neointima coverage was classified into three grades (0: no coverage; 1: poor coverage; 2: complete coverage), as previously reported.11 ,12 Yellow colour was classified into four grades (0: white; 1: slight yellow; 2: yellow; 3: intensive yellow) compared with standard colours, as previously reported.27 Thrombus was defined as white or red material with a cotton-like or ragged appearance or fragmentation with or without protrusion into the lumen or adherent to the luminal surface. Maximum and minimum neointima coverage grade, maximum yellow colour grade and the presence or absence of thrombus were determined for each stented segment. The neointima heterogeneity index was calculated as maximum − minimum coverage grade. In the present study, poor healing in the neointima coverage after stenting was judged by a high incidence of thrombus, and advanced atherosclerosis with high vulnerability was judged by a high yellow colour grade. Two angioscopy specialists blinded to the characteristics of the patients evaluated the angioscopic images. In cases of disagreement, a third reviewer served as an arbitrator. The inter- and intra-observer reproducibility for the interpretation of angioscopic images was 95% and 95% for stent coverage, 85% and 95% for plaque colour, and 90% and 100% for thrombus, respectively.
Statistical analysis
Continuous data were presented as mean±SD. Comparisons were made between groups by the unpaired Student t test, χ2 test or Mann–Whitney test. A p value <0.05 was regarded as statistically significant. Analysis was performed with SPSS V.16.0 J for Windows (SPSS, Chicago, Illinois, USA).
Results
Patient and lesion characteristics
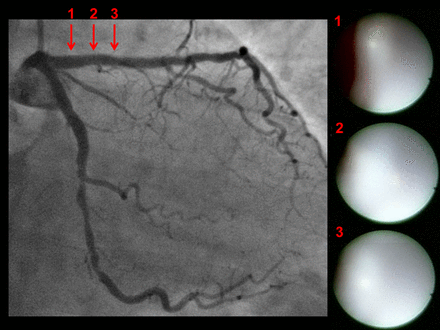
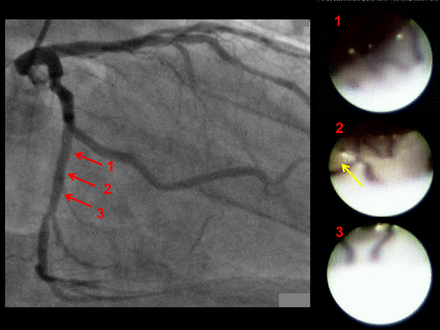
Included were 45 lesions in 45 patients for ZES and 45 lesions in 45 patients for EES; four patients with ZES and five patients with EES were excluded due to restenosis. Follow-up intervals were 371±61 days for ZES and 366±33 days for EES. There was no significant difference in the characteristics of the patients or the lesions between the groups (table 1), except that ZES were implanted more in the right coronary artery than EES and the stent size was larger in ZES than in EES. Representative cases of ZES and EES are shown in figures 1 and 2.
Patient and lesion characteristics
Representative case with zotarolimus-eluting stents (ZES): angiographic and angioscopic images 1 year after implantation of ZES in the proximal left anterior descending coronary artery. No in-stent restenosis was detected by angiography. Angioscopy shows that stent struts were completely buried under white thick neointima (grade 2 coverage) and no thrombus was detected on the neointima.
Representative case with everolimus-eluting stents (EES): angiographic and angioscopic images 1 year after implantation of EES in the proximal left circumflex coronary artery. No in-stent restenosis was detected by angiography. Angioscopy shows stent struts were observed on the vessel wall but were covered by a thin layer (grade 1 coverage). A yellow plaque was observed behind the stent (yellow arrow in angioscopic image 2). However, no thrombus was detected in the stented segment.
Angioscopic findings
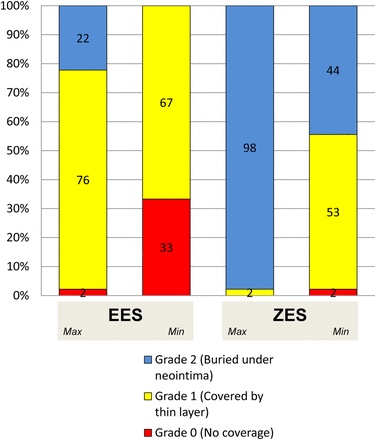
Maximum (2.0±0.2 vs 1.2±0.5, p<0.001) and minimum (1.5±0.6 vs 0.7±0.5, p<0.001) neointima coverage grade was higher in ZES than in EES. Neointima coverage was generally homogeneous in both groups and the neointima heterogeneity index was not different between ZES and EES (0.4±0.5 vs 0.5±0.6, p=0.42). The distribution of the neointima coverage grade is shown in figure 3. One-third (33%) of EES had no neointima coverage (grade 0) compared with only one (2%) ZES.
Distribution of neointima coverage grade. Maximum (2.0±0.2 vs 1.2±0.5, p<0.001) and minimum (1.5±0.6 vs 0.7±0.5, p<0.001) stent coverage was better with zotarolimus-eluting stents (ZES) than with everolimus-eluting stents (EES). Neointima coverage was generally homogeneous in both groups and the neointima heterogeneity index did not different between ZES and EES (0.4±0.5 vs 0.5±0.6, p=0.42). Grade 2 coverage was detected more frequently in ZES than in sirolimus-eluting stent (SES) (98% vs 22%, p<0.001), and grade 0 coverage was detected more frequently in SES than in ZES (33% vs 2%, p<0.001).
The incidence of thrombus was very low and was not different between ZES and EES (2% vs 4%, p=0.55).
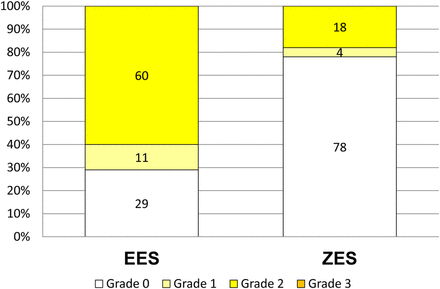
Maximum yellow colour grade was higher in EES than in ZES (1.3±0.9 vs 0.4±0.8, p<0.001). The distribution of maximum yellow colour grade is shown in figure 4.
Distribution of maximum yellow colour grade. Maximum yellow colour grade was higher in everolimus-eluting stents (EES) than in zotarolimus-eluting stents (ZES) (1.3±0.9 vs 0.4±0.8, p<0.001). White (grade 0) neointima was detected more frequently in ZES than in sirolimus-eluting stent (78% vs 29%, p<0.001).
Cases in which the majority (>50%) of the stent was covered by white and complete (grade 2) neointima coverage was more common in ZES than in EES (98% vs 16% p<0.001).
Discussion
We have compared the in-stent incidence of thrombus and the extent of atherosclerosis between ZES and EES at 1 year after implantation. Neointima was generally homogeneous and the incidence of thrombus was remarkably low in both DES, suggesting good healing after stent implantation with both. However, the maximum yellow colour grade at the stented segment was significantly higher in EES than in ZES, suggesting the presence of more advanced atherosclerosis in EES than in ZES.
Delayed healing as a cause of late stent failure
The incidence of thrombus at follow-up in SES has been reported to be 10–40%.11–19 This delayed healing or lack of healing with a high frequency of thrombogenesis that lasts for years would be an important mechanism for thrombotic occlusion or stenosis progression in first-generation DES. However, as demonstrated in the present study, both ZES and EES had a very low incidence of thrombus and therefore delayed healing is not a major mechanism for late stent failure with these stents.
Although the neointima coverage in ZES appeared to be as good as that in BMS, the neointima coverage in EES appeared to be as poor as in SES when compared with previous studies.11–19 Indeed, one-third (33%) of EES had grade 0 coverage area compared with only one (2%) of ZES. However, the thin but homogeneous neointima in EES might have contributed to the good healing with a low incidence of thrombus.
Neoatherosclerosis as a cause of late stent failure
The development of atherosclerotic plaque and its disruption (ie, neoatherosclerosis) has been known as a cause of acute coronary syndrome after BMS implantation.28 It usually takes about 5–10 years for the formation of atherosclerotic yellow plaque in the healthy white (non-atherosclerotic and fibrous) thick neointima that is commonly observed 1 year after BMS implantation and for the occurrence of acute coronary syndrome by the disruption of yellow plaque. The occurrence of atherosclerotic change in the neointima has been detected earlier in DES than in BMS by pathological studies.29 ,30 Formation of yellow plaque in the neointima was extremely rare within 1 year after BMS implantation but was frequently detected after SES implantation.12 Thus, DES (especially SES) is known to accelerate the progression of atherosclerosis. Furthermore, vulnerable plaques that had been present since before DES implantation would remain just behind the stent and easily progress to disrupt and cause acute coronary syndrome, but these plaques would be buried under thick white neointima when BMS was implanted. The progression of already present vulnerable plaques may also be a type of neoatheroslcerosis that causes late stent failure. When the neointima is very thin we cannot differentiate between yellow plaques in the original vessel wall behind the stent and those in the neointima, so the yellow colour of the stented lesions in the present study includes both of them.
According to the results of clinical trials with long-term follow-up of up to 5 years available in previous reports or at the website ClinicalTrial.gov, TLR at 1 and 5 years is 4.9% and 9.4% in SES,2 ,3 4.4% and 9.1% in paclitaxel-eluting stent (PES),4 ,5 5.9% and 7.5% in ZES6 ,7 and 3.4% and 8.9% in EES.8 Late stent failure as shown by the yearly TLR between 1 and 5 years is therefore 1.1%/year, 1.2%/year, 0.4%/year and 1.4%/year with SES, PES, ZES and EES, respectively. According to a recent report from the ENDEAVOR III trial, although higher angiographic restenosis was observed in ZES than in SES at the 9-month follow-up, cumulative outcomes through 5 years demonstrated that the composite endpoint of major adverse cardiac events and the important components of death, as well as cardiac death and myocardial infarction, favoured treatment with ZES compared with SES.31 Further investigation is required to clarify the association between the presence of in-stent yellow plaque and the incidence of late stent failure.
To prevent late stent failure caused by unhealed lesion that is already thrombogenic at 1 year, antiplatelet therapy would be important; however, to prevent late stent failure caused by new disruption of vulnerable plaque in the future, anti-atherosclerotic therapy including statin treatment may be more important. This hypothesis is expected to be tested in future investigations.
Study limitations
Although the patient characteristics were generally similar between ZES and EES, they were not completely matched as this was not a randomised trial. This was a single-time observational study at 1 year after implantation so the baseline angioscopic data at the time of stent implantation were not available. A randomised trial or a serial follow-up study would be required to confirm the results of the present study. Although there is no angioscopy-pathology validation study to confirm that yellow plaque in the neointima is an atherosclerotic lesion, since angioscopy is a device only to visualise the vessel wall by full-colour real-time image, we can translate the image using the knowledge of macroscopic pathology.
Conclusions
Although both ZES and EES had good healing with homogeneous neointima coverage and a low incidence of thrombus formation, EES had more advanced atherosclerosis with higher grade yellow plaque than ZES at 1 year after implantation.
References
Footnotes
-
Contributors All authors contributed to the design of the study, interpretation of the data and critical reviewing for writing the manuscript. KM and YU are the principal investigators and planned the protocol and performed the study with the help and valuable suggestions of MN, AH, MA, TN, AM, and KK. KK is the supervisor of Osaka Police Hospital and contributed to important discussion in the present study. All authors have read and approved the manuscript.
-
Competing interests None.
-
Ethics approval The study was approved by the Osaka Police Hospital Ethical Committee.
-
Patient consent Obtained.
-
Provenance and peer review Not commissioned; externally peer reviewed.