Article Text
Abstract
The introduction of drug-eluting stents (DESs) and superior anticoagulation has successfully improved the safety and patency rates of complex percutaneous coronary interventions (PCIs). The evolving techniques of contemporary PCI have been unable to completely eliminate coronary injury and mechanical complications. Primary causes for abrupt closure include dissection, thrombus formation and acute stent thrombosis. Initial treatment for abrupt closure includes balloon redilatation, optimisation of activated clotting time (ACT) and deployment of stent to stabilise a dissection. Coronary perforation is one of the most challenging and feared complications of PCI. It is most frequently due to distal wire or balloon/stent oversizing and should be fixed with balloon occlusion. Covered stent may be needed for large perforation in major proximal vessels. Perforations in small or distal vessels not resolving with balloon occlusion may be managed by coil or Gelfoam embolisation. Referral to emergency coronary artery bypass surgery (CABG) should be an option in case perforations do not seal.
Statistics from Altmetric.com
Introduction
It is a painful thing to look at your trouble and know that you yourself and no-one else had made it.
Ajax. Sophocles, 447 BC.
Due to evolution of drug-eluting stent (DES) and superior pharmacotherapy, contemporary percutaneous coronary intervention (PCI) is associated with increased patency and relatively low risk of complications. While less frequent than in the past, major complications such as death (0.7%) and myocardial infarction (MI) (2%) still occur in these procedures.1 Randomised trials have not provided good evidence for blanket recommendation, and avoidance of these feared complications of PCI is accomplished by operator experience and appropriate preventive techniques. This review explores the most common procedural complications like dissection, abrupt closure and coronary perforation.
Abrupt closure
Incidence
The incidence of abrupt closure during PCI has decreased from 3% in the balloon angioplasty era to 0.3% in the current era. This decreasing incidence corresponds to the increased use of stents and effective antithrombotics.1
Mechanisms
The common mechanism of abrupt closure is dissection followed by thrombus formation.2
Vasoconstriction is a rare mechanism of closure.3 The cause is indeterminate in almost 50% of patients.4 ,5 Patient-related factors of abrupt closure include unstable angina, multivessel disease, female gender and chronic renal failure.6 ,7 Angiographic risk factors predictive of abrupt closure are proximal tortuosity, diffuse lesion, pre-existing thrombus, degenerated vein graft and extremely angulated lesion.7 In the DES era, common causes of abrupt closure are stent edge dissection and acute stent thrombosis.
Management
Abrupt closure results in acute ischaemia manifesting as ECG changes, hypotension, bradycardia, chest pain and ventricular arrhythmias. The priority lies in stabilising hemodynamics and relieving ischaemia. Vasopressors, inotropes and intra-aortic balloon pump (IABP) should be considered for unstable hemodynamics. Atropine, intravenous fluid and vasopressors may be considered to address hypotension and bradycardia. Arrhythmias should be treated with antiarrhythmic drugs and cardioversion. Prompt balloon inflation should be attempted to establish antegrade flow. Urgent stenting is useful for stabilising the dissection. Glycoprotein IIb/IIIa antagonists may be helpful if acute closure is due to thrombus. Control of anticoagulation is of paramount importance to avoid thrombotic occlusion of stented artery. Activated clotting time (ACT) should be measured at intervals of 30 min to avoid overdosing and underdosing. For patients who receive weight-based bivalirudin infusion, ACT should be measured with 10 min of the infusion and the initial bolus to confirm appropriate drug delivery. With persistent abrupt closure, intravascular ultrasound (IVUS) may demonstrate the presence and extent of dissection. Multiple stenting may be required depending on the extent of dissection. Aspiration thrombectomy and glycoprotein IIb/IIIa inhibitors should be considered for thrombus-laden lesion. Patients with a successful outcome require close monitoring in an intensive care setting. If recanalisation is unsuccessful, the patient may be referred for an emergency coronary artery bypass surgery (CABG) or medical treatment (table 1).
Causes and treatment of abrupt closure
Dissection
A coronary artery dissection refers to split or a tear in the wall of the artery, which compresses or compromises the lumen of the artery reducing blood flow. Even if it's incidence requiring emergency CABG in the stent era is less than 0.2%,8 this complication should be recognised initially as treatment improves vessel patency and patient outcome. In the National Heart, Lung and Blood Institute scheme, dissection is defined as an intraluminal filling defect or flap associated with hazy, ground glass appearance. The classic types A–F classification remains useful to describe the severity of luminal injury (table 2).2 In the current era, main causes of iatrogenic coronary dissection are guide catheter-induced dissection, spiral dissection and stent edge dissection.
Classification of coronary dissection
Guide catheter dissection
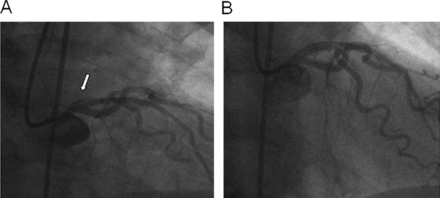
Guide catheter dissection, which is seen in less than 1% of PCI, is associated with deep engagement of large catheters into smaller, diseased arteries. Proximal dissection can cause abrupt closure and is the most common indication for emergency CABG. Conservative management may be successful in patients with localised dissection.9 When left main coronary artery (LMCA) dissection is recognised, immediate navigation of guidewires into the major bifurcation vessels of the LMCA should be attempted. A different catheter curve is recommended if the true lumen cannot be wired. A single stent should be deployed for localised LMCA dissection (figure 1). If the dissection propagates into a major branch artery, a stent should be placed directly into that artery while protecting the other branches with guidewires. After stenting all major arteries, repeat angiography in multiple views should be done prior to removal of guiding catheter.
Left main coronary artery dissection. (A) Catheter-induced dissection. (B) Stenting of left main sealing the dissection.
This complication can be minimised by avoiding deep engagement of guide catheter, pulling it back while any device is withdrawn from the artery and by not keeping it too long in deep engagement position inside LMCA.
Spiral dissection
While spiral dissection is rare in contemporary PCI, it can be spontaneous or iatrogenic. Iatrogenic dissection is much more common, which may result from forceful contrast injection into an unrecognised tissue plane following catheter-induced injury. Once recognised, a coronary wire should be advanced into the true lumen followed by rapid stenting. IVUS can confirm if the guidewire has entered the true lumen or the false lumen during navigation through the dissection planes. Stenting of the entire dissected segment has been performed with good angiographic result. Retrograde aortic dissection secondary to coronary dissection is a rare complication with incidence of 0.02%, but can be a nightmare for any operator. It must be quickly ruled out when there is unexplained chest pain or hypotension after angioplasty or stenting of any ostial or proximal lesion. The extent of the dissection will determine the approach used in the management of this complication. Extension of the dissection into aortic root of less than 40 mm can be managed by watchful expectancy or stenting of the coronary ostium. Surgical consultation is needed when there is significant aortic regurgitation, involvement of the supra-aortic vessels and progression of the index dissection. Dissection extending beyond 40 mm into aortic root requires surgery. Follow-up CT scan of the chest may identify the medically stabilised patient needing no further treatment or the patient with complications who may require surgery.10
Stent edge dissection
The exact mechanism of stent edge dissection is unknown, but it has been hypothesised that a false lumen is created after initial disruption from stent struts deployed at high pressure.11
Conservative management should be recommended for small, localised dissections when additional stent is contraindicated. Stenting should be considered if tissue flap is apparent on IVUS or for any flow limitation.
In order to prevent this dissection, usually the patient would have low-pressure 6–8 atm balloon predilatation. However, in cases of heavy calcification, a high-pressure small size balloon with the same size as predicted stent could be recommended both for predilatation (low pressure) and postdilatation (high pressure), so that the stent would not be oversized too much to cause dissection.
Coronary perforation
Incidence
Coronary perforation, defined as evidence of extravasation of contrast medium or blood from the coronary artery, is a serious complication with an incidence of less than 1.0%.12–20
It is responsible for 20% of cases referred for emergency CABG.21 Lesions associated with perforation are more complex in nature like American College of Cardiology type B or C, calcified lesions or chronic total occlusion (CTO).15 ,22 Women and elderly are more likely to sustain perforation.4
Mechanism
Coronary perforation is induced mainly due to guidewire penetration and vessel rupture. Recanalisation of CTO has become a common setting for perforation, usually due to small guidewire perforations with increasing use of stiffer and hydrophilic guidewires.22 Vessel rupture is usually induced by balloon or stent oversizing. Balloon to artery ratio more than 1.2 : 1 increases the risk of perforation.4 Sometimes, with extensive dissection or calcification, even the use of an appropriately sized balloon catheter may result in perforation. Use of a debulking procedure such as rotational atherectomy also increases the risk of perforation.23 Various angiographic factors predictive of perforation have been described (table 3).24 Ellis-graded perforations (table 4) fall into severity, ranging from small endovascular leaks into the adventia (grade I) to frank extravasation into the pericardial space (grade III). Grade I perforation is frequently caused by guidewires and rarely by debulking devices as well as stents. Occasionally, stiff and hydrophilic guidewires can cause large distal perforations in tortuous vessels. Grades II and III perforations are usually caused by high-pressure balloon inflations, oversized balloon catheters or stents or the use of debulking devices. There are no convincing data suggesting that glycoprotein IIb/IIIa inhibitors increase the frequency or the risk of perforation.25
Risk factors for coronary perforations
Classification of coronary perforation
Prognosis
Perforation severity correlates with worsening severity as mentioned by Ellis.13 Grade III perforations can quickly result in cardiac tamponade, rapid hemodynamic collapse, MI and/or death.4 ,12 ,14–16 ,22 ,23 ,26
Diagnosis
The diagnosis of perforation is made by coronary angiography. The patient may have severe chest pain, dizziness, nausea and vomiting out of proportion to that typically observed with balloon inflation. There may be persistent ST segment changes after balloon inflation. Severe bradycardia and hypotension may accompany perforations.27 Delayed cardiac tamponade up to 24- hours post-PCI can occur and demands awareness and vigilance of the interventionist.4 ,28
Prevention
To avoid perforation, the tip of a guidewire is advanced gently without forcing against resistance. It should move freely. Once in the distal segment, the interventionist must avoid placing the tip in small branches.
After inflation of a balloon, the deflated balloon should be kept in place; ECG should be watched to see if it reverses to baseline. The patient should be asked if there is relief of chest pain after balloon deflation. With a small contrast injection, if there is good distal flow without obvious extravasation of blood, then the balloon should be pulled back in the guidewire to be reinflated should perforation occur (table 5).
Prevention of coronary perforation
Treatment
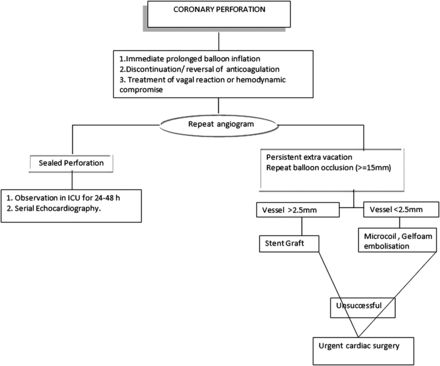
Grade I perforation that does not progress can be managed conservatively and antiplatelet agents can be continued if staining is not seen following stenting. This type of perforation with large oozing is treated with reversal of anticoagulation and/or prolonged balloon inflation at or proximal to the perforated segment. Guidewire perforation is best treated with balloon occlusion, but can also be treated with the delivery of occlusive coils, fat or beads. In the most severe cases, such as large grade II and grade III perforations, culprit artery occlusion is required while other measures like pericardiocentesis, deployment of polytetrafluoroehylene (PTFE) covered stent and referral for surgery would be needed on case-by-case basis.
Once perforation is recognised, the first step is to advance a balloon from the guide catheter and inflate it proximal to or over the site of perforation to occlude the flow (prolonged inflation of 10–30 min usually at 2–4 atm). Sometimes aggressive treatment with intravenous fluids, atropine, vasopressors, and an IABP may be necessary. Anticoagulation is immediately discontinued. In case of life-threatening bleeds with perforation, reversal with protamine is usually indicated if heparin was used as anticoagulant. Glycoprotein IIb/IIIa infusion should be stopped and abciximab must be reversed with infusion of platelets. The action of eptifibatide and tirofiban cannot be reversed with platelet infusions, but they have a shorter half-life. Bivaluridin is increasingly used for peri-PCI anticoagulation, and the short half-life of this agent is advantageous in sealing wire perforations. Infusion of fresh frozen plasma is the only means of reversing anticoagulation with bivaluridin.
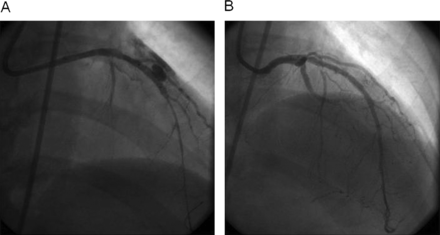
Emergency pericardiocentesis is indicated if a large pericardiac effusion is associated with cardiac tamponade physiology. Either a right heart catheter or fluoroscopy of the right heart's border may be useful to diagnose tamponade. A major advance in the treatment of coronary perforation is the availability of PTFE membrane-covered stents (figure 2). The stent graft excludes the perforation, possibly at the cost of occluding side branches. The device is rigid and delivery into tortuous vessels can be challenging. As most guide catheters cannot accommodate angioplasty balloon and stent graft, a dual guide technique has been developed wherein contralateral access is established and a separate guide catheter is used to deliver the stent. A second guidewire is advanced just proximal to the occluding balloon, which is then deflated and retracted, allowing passage of a new guidewire and covered stent for complete closure of the perforation. This technique employing two guide catheters has been documented to decrease the rate of adverse events.29
Grade III perforation in a native left anterior descending coronary artery causing tamponade. (A) Left interior descending coronary artery perforation in an 80-year-old gentleman with 75% lesion. A drug-eluting stent was deployed directly at the lesion causing a large grade III perforation with contrast extravasation in the pericardial space. (B) A Jomed covered stent (Jomed International AB, Helsingborg, Sweden) was successfully deployed sealing the perforation.
Side branch near the perforation site may be excluded by covered stent, which might result in periprocedural MI.30 This dual stent layer should be dilated aggressively (>18 atm) and judiciously. IVUS may be used to verify adequate stent expansion. Prior to the advent of covered stents, grade III perforation required emergency CABG, which carried significant mortality.28 Perforations in small vessels can be addressed with either pronged balloon occlusion or the injection of thrombin, polyvinyl alcohol, Gelfoam, collagen or embolisation of microcoils or beads (figure 3).31 ,32
Algorithm for management of coronary perforation.27
Conclusion
Contemporary PCI is associated with a relatively low risk of dissection, abrupt closure and perforation. Randomised trials have not provided good evidence for blanket recommendations, and avoidance of these feared complications of PCI is best accomplished by operator experience and preventive approaches. Prevention is always the first priority because it is better to stay out of trouble rather than to get out of it. Continued education in the evolving field of PCI will allow further improvement in patient outcomes.
References
Footnotes
-
Competing interests None.
-
Provenance and peer review Not commissioned; internally peer reviewed.